Nutrient supply and nucleus pulposus cell function: effects of the transport properties of the cartilage endplate and potential implications for intradiscal biologic therapy
- PMID: 30721733
- PMCID: PMC6536352
- DOI: 10.1016/j.joca.2019.01.013
Nutrient supply and nucleus pulposus cell function: effects of the transport properties of the cartilage endplate and potential implications for intradiscal biologic therapy
Abstract
Objective: Intradiscal biologic therapy is a promising strategy for managing intervertebral disc degeneration. However, these therapies require a rich nutrient supply, which may be limited by the transport properties of the cartilage endplate (CEP). This study investigated how fluctuations in CEP transport properties impact nutrient diffusion and disc cell survival and function.
Design: Human CEP tissues harvested from six fresh cadaveric lumbar spines (38-66 years old) were placed at the open sides of diffusion chambers. Bovine nucleus pulposus (NP) cells cultured inside the chambers were nourished exclusively by nutrients diffusing through the CEP tissues. After 72 h in culture, depth-dependent NP cell viability and gene expression were measured, and related to CEP transport properties and biochemical composition determined using fluorescence recovery after photobleaching and Fourier transform infrared (FTIR) spectroscopy.
Results: Solute diffusivity varied nearly 4-fold amongst the CEPs studied, and chambers with the least permeable CEPs appeared to have lower aggrecan, collagen-2, and matrix metalloproteinase-2 gene expression, as well as a significantly shorter viable distance from the CEP/nutrient interface. Increasing chamber cell density shortened the viable distance; however, this effect was lost for low-diffusivity CEPs, which suggests that these CEPs may not provide enough nutrient diffusion to satisfy cell demands. Solute diffusivity in the CEP was associated with biochemical composition: low-diffusivity CEPs had greater amounts of collagen and aggrecan, more mineral, and lower cross-link maturity.
Conclusions: CEP transport properties dramatically affect NP cell survival/function. Degeneration-related CEP matrix changes could hinder the success of biologic therapies that require increased nutrient supply.
Keywords: Cartilage endplate; Disc degeneration; Fourier transform infrared imaging; Low back pain; Nucleus pulposus cell; Nutrient transport.
Copyright © 2019 Osteoarthritis Research Society International. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest
None.
Figures



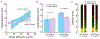


References
-
- Wang Z, Hutton WC, Yoon ST. ISSLS Prize winner: Effect of link protein peptide on human intervertebral disc cells. Spine 2013;38:1501–1507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

