A report of activities related to the Dietary Reference Intakes from the Joint Canada-US Dietary Reference Intakes Working Group
- PMID: 30721931
- PMCID: PMC6500900
- DOI: 10.1093/ajcn/nqy293
A report of activities related to the Dietary Reference Intakes from the Joint Canada-US Dietary Reference Intakes Working Group
Abstract
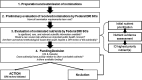
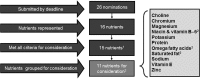
The governments of the United States and Canada have jointly undertaken the development of the Dietary Reference Intakes (DRIs) since the mid-1990s. The Federal DRI committees from each country work collaboratively to identify DRI needs, prioritize nutrient reviews, advance work to resolve methodological issues that is necessary for new reviews, and sponsor DRI-related committees through the National Academies of Sciences, Engineering and Medicine. In recent years, the Joint Canada-US DRI Working Group, consisting of members from both Federal DRI committees, developed an open and transparent nomination process for prioritizing nutrients for DRI review, by which sodium, the omega-3 (n-3) fatty acids, vitamin E, and magnesium were identified. In addition, discussions during the nutrient nomination process prompted the Federal DRI committees to address previously identified issues related to the use of chronic disease endpoints when setting DRIs. The development of guiding principles for setting DRIs based on chronic disease risk reduction will be applied for the first time during the DRI review of sodium and potassium. In summary, the US and Canadian governments have worked collaboratively to adapt our approach to prioritizing nutrients for DRI review and to broaden the scope of the DRIs to better incorporate the concept of chronic disease risk reduction in order to improve public health.
Figures

References
-
- Institute of Medicine. How Should the Recommended Dietary Allowances be Revised?. Washington (DC): The National Academies Press; 1994. - PubMed
-
- Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington (DC): The National Academies Press; 2006.
-
- Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington (DC): The National Academies Press; 1997. - PubMed
-
- Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington (DC): The National Academies Press; 1998. - PubMed
-
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington (DC): The National Academies Press; 2000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

