A multicountry randomized controlled trial of comprehensive maternal nutrition supplementation initiated before conception: the Women First trial
- PMID: 30721941
- PMCID: PMC6367966
- DOI: 10.1093/ajcn/nqy228
A multicountry randomized controlled trial of comprehensive maternal nutrition supplementation initiated before conception: the Women First trial
Abstract
Background: Reported benefits of maternal nutrition supplements commenced during pregnancy in low-resource populations have typically been quite limited.
Objectives: This study tested the effects on newborn size, especially length, of commencing nutrition supplements for women in low-resource populations ≥3 mo before conception (Arm 1), compared with the same supplement commenced late in the first trimester of pregnancy (Arm 2) or not at all (control Arm 3).
Methods: Women First was a 3-arm individualized randomized controlled trial (RCT). The intervention was a lipid-based micronutrient supplement; a protein-energy supplement was also provided if maternal body mass index (kg/m2) was <20 or gestational weight gain was less than recommendations. Study sites were in rural locations of the Democratic Republic of the Congo (DRC), Guatemala, India, and Pakistan. The primary outcome was length-for-age z score (LAZ), with all anthropometry obtained <48 h post delivery. Because gestational ages were unavailable in DRC, outcomes were determined for all 4 sites from WHO newborn standards (non-gestational-age-adjusted, NGAA) as well as INTERGROWTH-21st fetal standards (3 sites, gestational age-adjusted, GAA).
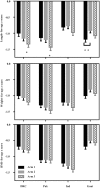
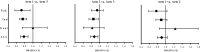
Results: A total of 7387 nonpregnant women were randomly assigned, yielding 2451 births with NGAA primary outcomes and 1465 with GAA outcomes. Mean LAZ and other outcomes did not differ between Arm 1 and Arm 2 using either NGAA or GAA. Mean LAZ (NGAA) for Arm 1 was greater than for Arm 3 (effect size: +0.19; 95% CI: 0.08, 0.30, P = 0.0008). For GAA outcomes, rates of stunting and small-for-gestational-age were lower in Arm 1 than in Arm 3 (RR: 0.69; 95% CI: 0.49, 0.98, P = 0.0361 and RR: 0.78; 95% CI: 0.70, 0.88, P < 0.001, respectively). Rates of preterm birth did not differ among arms.
Conclusions: In low-resource populations, benefits on fetal growth-related birth outcomes were derived from nutrition supplements commenced before conception or late in the first trimester. This trial was registered at clinicaltrials.gov as NCT01883193.
Figures


Comment in
-
Balancing the benefits of maternal nutritional interventions; time to put women first!Am J Clin Nutr. 2019 Feb 1;109(2):249-250. doi: 10.1093/ajcn/nqy336. Am J Clin Nutr. 2019. PMID: 30721972 No abstract available.
-
Comments on "A multicountry randomized controlled trial of comprehensive maternal nutrition supplementation initiated before conception: the Women First trial".Am J Clin Nutr. 2019 Aug 1;110(2):526-527. doi: 10.1093/ajcn/nqz106. Am J Clin Nutr. 2019. PMID: 31367758 No abstract available.
-
Response to Editorial: Balancing the benefits of maternal nutritional interventions; time to put women first!Am J Clin Nutr. 2019 Aug 1;110(2):521-522. doi: 10.1093/ajcn/nqz077. Am J Clin Nutr. 2019. PMID: 31367762 Free PMC article. No abstract available.
-
Reply to NF Krebs and KM Hambidge.Am J Clin Nutr. 2019 Aug 1;110(2):521-522. doi: 10.1093/ajcn/nqz078. Am J Clin Nutr. 2019. PMID: 31367763 No abstract available.
-
Reply to D Flood et al.Am J Clin Nutr. 2019 Aug 1;110(2):527-528. doi: 10.1093/ajcn/nqz107. Am J Clin Nutr. 2019. PMID: 31367765 No abstract available.
References
-
- UNICEF. Tracking progress on child and maternal nutrition: a survival and development priority. New York, NY: UNICEF; 2009.
-
- World Health Organization. Global nutrition targets 2025: Policy Brief Series (WHO/NMH/NH/14.2)[Internet]. 2014;2017 [cited June, 2018] Available from: http://www.who.int/nutrition/publications/globaltargets2025_policybrief_....
-
- World Bank. World Bank annual report 2016. Washington (DC): World Bank; 2016.
-
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–60. - PubMed
-
- Victora CG, de Onis M, Hallal PC, Blossner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatr. 2010;125(3):e473–80. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

