Antibodies Against Egg- and Cell-Grown Influenza A(H3N2) Viruses in Adults Hospitalized During the 2017-2018 Influenza Season
- PMID: 30721982
- PMCID: PMC6534188
- DOI: 10.1093/infdis/jiz049
Antibodies Against Egg- and Cell-Grown Influenza A(H3N2) Viruses in Adults Hospitalized During the 2017-2018 Influenza Season
Abstract
Background: Influenza vaccine effectiveness was low in 2017-2018, yet circulating influenza A(H3N2) viruses were antigenically similar to cell-grown vaccine strains. Notably, most influenza vaccines are egg propagated.
Methods: Serum specimens were collected shortly after illness onset from 15 influenza A(H3N2) virus-infected cases and 15 uninfected hospitalized adults. Geometric mean titers against egg- and cell-grown influenza A/Hong Kong/4801/2014(H3N2) virus vaccine strains and representative circulating viruses (including A/Washington/16/2017) were determined by a microneutralization (MN) assay. Independent effects of strain-specific titers on susceptibility were estimated by logistic regression.
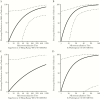
Results: MN titers against egg-grown influenza A/Hong Kong virus were significantly higher among vaccinated individuals (173 vs 41; P = 0.01). In unadjusted models, a 2-fold increase in titers against egg-grown influenza A/Hong Kong virus was not significantly protective (29% reduction; P = .09), but a similar increase in the cell-grown influenza A/Washington virus antibody titer (3C.2a2) was protective (60% reduction; P = .02). Higher egg-grown influenza A/Hong Kong virus titers were not significantly associated with infection, when adjusted for antibody titers against influenza A/Washington virus (15% reduction; P = .61). A 54% reduction in the odds of infection was observed with a 2-fold increase in titer against influenza A/Washington virus (P = not significant), adjusted for the titer against egg-grown influenza A/Hong Kong virus titer.
Conclusion: Individuals vaccinated in 2017-2018 had high antibody titers against the egg-adapted vaccine strain and lower titers against circulating viruses. Titers against circulating but not egg-adapted strains were correlated with protection.
Keywords: Influenza A; antibodies; egg adaptation; vaccine effectiveness.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Age-specific effects of vaccine egg adaptation and immune priming on A(H3N2) antibody responses following influenza vaccination.J Clin Invest. 2021 Apr 15;131(8):e146138. doi: 10.1172/JCI146138. J Clin Invest. 2021. PMID: 33690218 Free PMC article.
-
Low population serum microneutralization antibody titer against the predominating influenza A(H3N2) N121K virus during the severe influenza summer peak of Hong Kong in 2017.Emerg Microbes Infect. 2018 Mar 6;7(1):23. doi: 10.1038/s41426-018-0041-1. Emerg Microbes Infect. 2018. PMID: 29511175 Free PMC article.
-
Neutralizing Antibody Responses to Antigenically Drifted Influenza A(H3N2) Viruses among Children and Adolescents following 2014-2015 Inactivated and Live Attenuated Influenza Vaccination.Clin Vaccine Immunol. 2016 Oct 4;23(10):831-839. doi: 10.1128/CVI.00297-16. Print 2016 Oct. Clin Vaccine Immunol. 2016. PMID: 27558294 Free PMC article.
-
Evaluation of influenza virus A/H3N2 and B vaccines on the basis of cross-reactivity of postvaccination human serum antibodies against influenza viruses A/H3N2 and B isolated in MDCK cells and embryonated hen eggs.Clin Vaccine Immunol. 2012 Jun;19(6):897-908. doi: 10.1128/CVI.05726-11. Epub 2012 Apr 4. Clin Vaccine Immunol. 2012. PMID: 22492743 Free PMC article.
-
H3N2 influenza viruses in humans: Viral mechanisms, evolution, and evaluation.Hum Vaccin Immunother. 2018;14(8):1840-1847. doi: 10.1080/21645515.2018.1462639. Epub 2018 May 14. Hum Vaccin Immunother. 2018. PMID: 29641358 Free PMC article. Review.
Cited by
-
Review of Analyses Estimating Relative Vaccine Effectiveness of Cell-Based Quadrivalent Influenza Vaccine in Three Consecutive US Influenza Seasons.Vaccines (Basel). 2022 Jun 3;10(6):896. doi: 10.3390/vaccines10060896. Vaccines (Basel). 2022. PMID: 35746504 Free PMC article.
-
Redirecting antibody responses from egg-adapted epitopes following repeat vaccination with recombinant or cell culture-based versus egg-based influenza vaccines.Nat Commun. 2024 Jan 4;15(1):254. doi: 10.1038/s41467-023-44551-x. Nat Commun. 2024. PMID: 38177116 Free PMC article. Clinical Trial.
-
Age-specific effects of vaccine egg adaptation and immune priming on A(H3N2) antibody responses following influenza vaccination.J Clin Invest. 2021 Apr 15;131(8):e146138. doi: 10.1172/JCI146138. J Clin Invest. 2021. PMID: 33690218 Free PMC article.
-
A rapid and flexible microneutralization assay for serological assessment of influenza viruses.Influenza Other Respir Viruses. 2023;17(4):e13141. doi: 10.1111/irv.13141. Influenza Other Respir Viruses. 2023. PMID: 37127782 Free PMC article.
-
Mapping person-to-person variation in viral mutations that escape polyclonal serum targeting influenza hemagglutinin.Elife. 2019 Aug 27;8:e49324. doi: 10.7554/eLife.49324. Elife. 2019. PMID: 31452511 Free PMC article.
References
-
- World Health Organization. Recommended composition of influenza virus vaccines for use in the 2018–2019 northern hemisphere influenza season 2018. http://www.who.int/influenza/vaccines/virus/recommendations/2018_19_nort.... Accessed 20 June 2018.
-
- Belongia EA, Simpson MD, King JP, et al. . Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis 2016; 16:942–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

