Compliance with ethical standards in the reporting of donor sources and ethics review in peer-reviewed publications involving organ transplantation in China: a scoping review
- PMID: 30723071
- PMCID: PMC6377532
- DOI: 10.1136/bmjopen-2018-024473
Compliance with ethical standards in the reporting of donor sources and ethics review in peer-reviewed publications involving organ transplantation in China: a scoping review
Abstract
Objectives: The objective of this study is to investigate whether papers reporting research on Chinese transplant recipients comply with international professional standards aimed at excluding publication of research that: (1) involves any biological material from executed prisoners; (2) lacks Institutional Review Board (IRB) approval and (3) lacks consent of donors.
Design: Scoping review based on Arksey and O'Mallee's methodological framework.
Data sources: Medline, Scopus and Embase were searched from January 2000 to April 2017.
Eligibility criteria: We included research papers published in peer-reviewed English-language journals reporting on outcomes of research involving recipients of transplanted hearts, livers or lungs in mainland China.
Data extraction and synthesis: Data were extracted by individual authors working independently following training and benchmarking. Descriptive statistics were compiled using Excel.
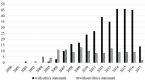
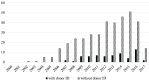
Results: 445 included studies reported on outcomes of 85 477 transplants. 412 (92.5%) failed to report whether or not organs were sourced from executed prisoners; and 439 (99%) failed to report that organ sources gave consent for transplantation. In contrast, 324 (73%) reported approval from an IRB. Of the papers claiming that no prisoners' organs were involved in the transplants, 19 of them involved 2688 transplants that took place prior to 2010, when there was no volunteer donor programme in China.
Discussion: The transplant research community has failed to implement ethical standards banning publication of research using material from executed prisoners. As a result, a large body of unethical research now exists, raising issues of complicity and moral hazard to the extent that the transplant community uses and benefits from the results of this research. We call for retraction of this literature pending investigation of individual papers.
Keywords: china; executed prisoners; organ donation; publication ethics; scoping review.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Dr AB is a member of the New Zealand Advocacy & Initiatives Committee (NZAIC) of the International Coalition to End Transplant Abuse in China, Dr BB has nothing to disclose, Dr RC has nothing to disclose, Dr RC-W is a member of the Australian Advocacy and Initiatives Committee of the International Coalition to End Transplant Abuse in China, Professor MFS is a member of the Ethics Committee of Doctors Against Forced Organ Harvesting, and a member of the Australian Advocacy and Initiatives Committee of the International Coalition to End Transplant Abuse in China, MPR reports that he is an occasional expert contributor to the International Coalition to End Transplant Abuse in China, Professor WR is a Director of the NGO ’International Coalition to End Transplant Abuse in China' and is chair of its International Advisory Committee.
Figures
Comment in
-
Nature's 10: Ten people who mattered in science in 2019.Nature. 2019 Dec;576(7787):361-372. doi: 10.1038/d41586-019-03749-0. Nature. 2019. PMID: 31848484 No abstract available.
References
-
- WMA - The World Medical Association. WMA Statement on organ and tissue donation. https://www.wma.net/policies-post/wma-statement-on-organ-and-tissue-dona... (Accessed 7 Feb 2018).
-
- Stock P. Policy and Ethics. The Transplantation Society. https://www.tts.org/about-tts-5/governance/policy-a-ethics (Accessed 7 Feb 2018).
-
- Amnesty International Publications. Amnesty International Report 2011: the State of the World’s Human Rights: Amnesty International Publications, 2011.
-
- The Declaration of Istanbul Custodian Group. The declaration of istanbul on organ trafficking and transplant tourism. http://www.declarationofistanbul.org/ (Accessed 7 Feb 2018).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous