Dominant activating RAC2 mutation with lymphopenia, immunodeficiency, and cytoskeletal defects
- PMID: 30723080
- PMCID: PMC6497516
- DOI: 10.1182/blood-2018-11-886028
Dominant activating RAC2 mutation with lymphopenia, immunodeficiency, and cytoskeletal defects
Abstract
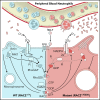
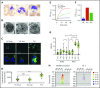
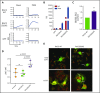
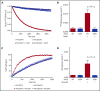
Ras-related C3 botulinum toxin substrate 2 (RAC2), through interactions with reduced NAD phosphate oxidase component p67 phox , activates neutrophil superoxide production, whereas interactions with p21-activated kinase are necessary for fMLF-induced actin remodeling. We identified 3 patients with de novo RAC2[E62K] mutations resulting in severe T- and B-cell lymphopenia, myeloid dysfunction, and recurrent respiratory infections. Neutrophils from RAC2[E62K] patients exhibited excessive superoxide production, impaired fMLF-directed chemotaxis, and abnormal macropinocytosis. Cell lines transfected with RAC2[E62K] displayed characteristics of active guanosine triphosphate (GTP)-bound RAC2 including enhanced superoxide production and increased membrane ruffling. Biochemical studies demonstrated that RAC2[E62K] retains intrinsic GTP hydrolysis; however, GTPase-activating protein failed to accelerate hydrolysis resulting in prolonged active GTP-bound RAC2. Rac2+/E62K mice phenocopy the T- and B-cell lymphopenia, increased neutrophil F-actin, and excessive superoxide production seen in patients. This gain-of-function mutation highlights a specific, nonredundant role for RAC2 in hematopoietic cells that discriminates RAC2 from the related, ubiquitous RAC1.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


References
-
- Knaus UG, Heyworth PG, Evans T, Curnutte JT, Bokoch GM. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991;254(5037):1512-1515. - PubMed
-
- Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM, Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7(3):202-210. - PubMed
-
- Abell AN, DeCathelineau AM, Weed SA, Ambruso DR, Riches DW, Johnson GL. Rac2D57N, a dominant inhibitory Rac2 mutant that inhibits p38 kinase signaling and prevents surface ruffling in bone-marrow-derived macrophages. J Cell Sci. 2004;117(Pt 2):243-255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

