Cyclin B3 promotes anaphase I onset in oocyte meiosis
- PMID: 30723090
- PMCID: PMC6446836
- DOI: 10.1083/jcb.201808091
Cyclin B3 promotes anaphase I onset in oocyte meiosis
Abstract
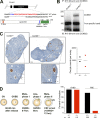
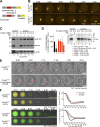
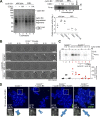
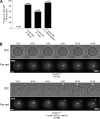
Meiosis poses unique challenges because two rounds of chromosome segregation must be executed without intervening DNA replication. Mammalian cells express numerous temporally regulated cyclins, but how these proteins collaborate to control meiosis remains poorly understood. Here, we show that female mice genetically ablated for cyclin B3 are viable-indicating that the protein is dispensable for mitotic divisions-but are sterile. Mutant oocytes appear normal until metaphase I but then display a highly penetrant failure to transition to anaphase I. They arrest with hallmarks of defective anaphase-promoting complex/cyclosome (APC/C) activation, including no separase activity, high CDK1 activity, and high cyclin B1 and securin levels. Partial APC/C activation occurs, however, as exogenously expressed APC/C substrates can be degraded. Cyclin B3 forms active kinase complexes with CDK1, and meiotic progression requires cyclin B3-associated kinase activity. Cyclin B3 homologues from frog, zebrafish, and fruit fly rescue meiotic progression in cyclin B3-deficient mouse oocytes, indicating conservation of the biochemical properties and possibly cellular functions of this germline-critical cyclin.
© 2019 Karasu et al.
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

