MYD88 L265P mutation and CDKN2A loss are early mutational events in primary central nervous system diffuse large B-cell lymphomas
- PMID: 30723112
- PMCID: PMC6373750
- DOI: 10.1182/bloodadvances.2018027672
MYD88 L265P mutation and CDKN2A loss are early mutational events in primary central nervous system diffuse large B-cell lymphomas
Abstract
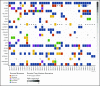
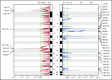
The genetic alterations that define primary central nervous system lymphoma (PCNSL) are incompletely elucidated, and the genomic evolution from diagnosis to relapse is poorly understood. We performed whole-exome sequencing (WES) on 36 PCNSL patients and targeted MYD88 sequencing on a validation cohort of 27 PCNSL patients. We also performed WES and phylogenetic analysis of 3 matched newly diagnosed and relapsed tumor specimens and 1 synchronous intracranial and extracranial relapse. Immunohistochemistry (IHC) for programmed death-1 ligand (PD-L1) was performed on 43 patient specimens. Combined WES and targeted sequencing identified MYD88 mutation in 67% (42 of 63) of patients, CDKN2A biallelic loss in 44% (16 of 36), and CD79b mutation in 61% (22 of 36). Copy-number analysis demonstrated frequent regions of copy loss (ie, CDKN2A), with few areas of amplification. CD79b mutations were associated with improved progression-free and overall survival. We did not identify amplification at the PD-1/PD-L1 loci. IHC for PD-L1 revealed membranous expression in 30% (13 of 43) of specimens. Phylogenetic analysis of paired primary and relapsed specimens identified MYD88 mutation and CDKN2A loss as early clonal events. PCNSL is characterized by frequent mutations within the B-cell receptor and NF-κB pathways. The lack of PD-L1 amplifications, along with membranous PD-L1 expression in 30% of our cohort, suggests that PD-1/PD-L1 inhibitors may be useful in a subset of PCNSL. WES of PCNSL provides insight into the genomic landscape and evolution of this rare lymphoma subtype and potentially informs more rational treatment decisions.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: P.K.B has received research funding from Merck and honorarium from Genentech and consulted for Lilly and Angiochem. T.T.B. has been a pharmaceutical consultant for Merck, NXDC, Amgen, and Proximagen/Upsher, served on a scientific advisory board for Genomicare, been a consultant for Jiahui Health and Champions Biotechnology, and been a contributor for Up to Date. The remaining authors declare no competing financial interests.
Figures

References
-
- Miller DC, Hochberg FH, Harris NL, Gruber ML, Louis DN, Cohen H. Pathology with clinical correlations of primary central nervous system non-Hodgkin’s lymphoma. The Massachusetts General Hospital experience 1958-1989. Cancer. 1994;74(4):1383-1397. - PubMed
-
- Camilleri-Broët S, Martin A, Moreau A, et al. . Primary central nervous system lymphomas in 72 immunocompetent patients: pathologic findings and clinical correlations. Groupe Ouest Est d’étude des Leucénies et Autres Maladies du Sang (GOELAMS). Am J Clin Pathol. 1998;110(5):607-612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

