A Phase II Randomized Study of Neoadjuvant Letrozole Plus Alpelisib for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer (NEO-ORB)
- PMID: 30723140
- PMCID: PMC6522303
- DOI: 10.1158/1078-0432.CCR-18-3160
A Phase II Randomized Study of Neoadjuvant Letrozole Plus Alpelisib for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer (NEO-ORB)
Abstract
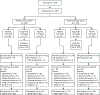
Purpose: Addition of alpelisib to fulvestrant significantly extended progression-free survival in PIK3CA-mutant, hormone receptor-positive (HR+) advanced/metastatic breast cancer in the phase III SOLAR-1 study. The combination of alpelisib and letrozole also had promising activity in phase I studies of HR+ advanced/metastatic breast cancer. NEO-ORB aimed to determine whether addition of alpelisib to letrozole could increase response rates in the neoadjuvant setting.Patients and Methods: Postmenopausal women with HR+, human epidermal growth factor receptor 2-negative, T1c-T3 breast cancer were assigned to the PIK3CA-wild-type or PIK3CA-mutant cohort according to their tumor PIK3CA status, and randomized (1:1) to 2.5 mg/day letrozole with 300 mg/day alpelisib or placebo for 24 weeks. Primary endpoints were objective response rate (ORR) and pathologic complete response (pCR) rate for both PIK3CA cohorts.
Results: In total, 257 patients were assigned to letrozole plus alpelisib (131 patients) or placebo (126 patients). Grade ≥3 adverse events (≥5% of patients) in the alpelisib arm were hyperglycemia (27%), rash (12%), and maculo-papular rash (8%). The primary objective was not met; ORR in the alpelisib versus placebo arm was 43% versus 45% and 63% versus 61% in the PIK3CA-mutant and wild-type cohorts, respectively. pCR rates were low in all groups. Decreases in Ki-67 were similar across treatment arms and cohorts. In PIK3CA-mutant tumors, alpelisib plus letrozole treatment induced a greater decrease in phosphorylated AKT versus placebo plus letrozole.
Conclusions: In contrast to initial results in advanced/metastatic disease, addition of alpelisib to 24-week neoadjuvant letrozole treatment did not improve response in patients with HR+ early breast cancer.
Trial registration: ClinicalTrials.gov NCT01923168.
©2019 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest:
I.A. Mayer has received consultancy/advisory fees from AstraZeneca, Novartis, Genentech, Eli Lilly, Immunomedics, MacroGenics, and GlaxoSmithKline, and received research funding from Novartis, Genentech, and Pfizer; A. Prat has received consultancy/advisory fees and received research funding from Nanostring Technologies, and received lecture fees from Novartis and Pfizer; D. Egle has received consultancy/advisory fees from AstraZeneca, Novartis, Pfizer, and Roche; J. Alejandro Perez Fidalgo has received consulting/advisory fees from Clovis and PharmaMar, and received speakers’ bureaus fees from AstraZeneca, Novartis, and Roche; M. Gnant has received consultancy/advisory fees from Accelsiors, Amgen, AstraZeneca, GlaxoSmithKline, Novartis, OBI Pharma, and Roche, and received research funding from AstraZeneca, Novartis, Pfizer, and Roche; P.A. Fasching has received grants/fees from Amgen, Celgene, Novartis, Pfizer, Puma Biotechnology, Roche, and Teva Pharmaceutical Industries; M. Colleoni has received consultancy/advisory fees from AstraZeneca, Celldex Therapeutics, Novartis, OBI Pharma, Pfizer, Pierre Fabre Laboratories, and Puma Biotechnology; A.C. Wolff has received research funding from Pfizer; E.P. Winer has received consultancy/advisory fees from Genentech, Leap Therapeutics, and Tesaro, and received research funding from Genentech and Merck; C.F. Singer has received consultancy/advisory fees from Amgen, AstraZeneca, Novartis, Pfizer, Roche, and Teva Pharmaceutical Industries, and received research funding from Roche and Novartis; S. Hurvitz has received research funding from Amgen, Bayer, Boehringer Ingelheim, Cascadian Therapeutics, Dignitana, Eli Lilly, Genentech/Roche, Merrimack Pharmaceuticals, Novartis, OBI Pharma, Pfizer, Puma Biotechnology, and Seattle Genetics; P.A. van Dam has received consultancy/advisory fees from Amgen, Novartis, and Roche, and received research funding from Amgen and Roche; S. Kuemmel has received consultancy/advisory fees from Amgen, AstraZeneca, Celgene, Daiichi Sankyo, Genomic Health, Novartis, Pfizer, Roche, and Teva Pharmaceutical Industries, and received research funding from Roche; C. Mundhenke has received consultancy/advisory and speakers’ bureaus fees from Novartis and Pfizer, and received research funding from Novartis; F. Holmes has received consultancy/advisory fees from Myriad Genetics, Novartis, and Puma Biotechnology; N. Babbar, L. Charbonnier, I Diaz-Padilla, and F.D. Vogl are employees of Novartis; D. Sellami is an employee and owns stocks/shares in Novartis; C.L. Arteaga receives or has received research funding from Bayer, Eli Lilly, Pfizer, Radius Health, and Takeda; he serves or has served in advisory roles to Symphogen, Daiichi Sankyo, TAIHO Oncology, Novartis, Merck, PUMA Biotechnology, Eli Lilly, Radius Health, Sanofi, OrigiMed, AbbVie, and H3 Biomedicine; he holds stock options in Provista and Y-TRAP. S. Blau and L. Garcia Estevez declare no conflicts of interest.
Figures

References
-
- National comprehensive cancer network clinical practice guidelines in oncology. Breast cancer. version 1. 2018. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. - PubMed
-
- Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer JU, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: The immediate preoperative anastrozole, tamoxifen, or combined with tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 2005;23:5108–16. - PubMed
-
- Cataliotti L, Buzdar AU, Noguchi S, Bines J, Takatsuka Y, Petrakova K, et al. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: The pre-operative “arimidex” compared to tamoxifen (PROACT) trial. Cancer 2006;106:2095–103. - PubMed
-
- Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: Clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype - ACOSOG Z1031. J Clin Oncol 2011;29:2342–9. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

