Characterization of the ATP4 ion pump in Toxoplasma gondii
- PMID: 30723156
- PMCID: PMC6462519
- DOI: 10.1074/jbc.RA118.006706
Characterization of the ATP4 ion pump in Toxoplasma gondii
Abstract
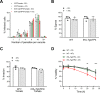
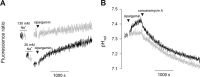
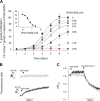
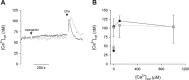
The Plasmodium falciparum ATPase PfATP4 is the target of a diverse range of antimalarial compounds, including the clinical drug candidate cipargamin. PfATP4 was originally annotated as a Ca2+ transporter, but recent evidence suggests that it is a Na+ efflux pump, extruding Na+ in exchange for H+ Here we demonstrate that ATP4 proteins belong to a clade of P-type ATPases that are restricted to apicomplexans and their closest relatives. We employed a variety of genetic and physiological approaches to investigate the ATP4 protein of the apicomplexan Toxoplasma gondii, TgATP4. We show that TgATP4 is a plasma membrane protein. Knockdown of TgATP4 had no effect on resting pH or Ca2+ but rendered parasites unable to regulate their cytosolic Na+ concentration ([Na+]cyt). PfATP4 inhibitors caused an increase in [Na+]cyt and a cytosolic alkalinization in WT but not TgATP4 knockdown parasites. Parasites in which TgATP4 was knocked down or disrupted exhibited a growth defect, attributable to reduced viability of extracellular parasites. Parasites in which TgATP4 had been disrupted showed reduced virulence in mice. These results provide evidence for ATP4 proteins playing a key conserved role in Na+ regulation in apicomplexan parasites.
Keywords: ATPase; Na+ pump; TgATP4; Toxoplasma gondii; cipargamin; drug action; malaria; membrane transport; protozoan parasite; sodium transporter.
© 2019 Lehane et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Blaustein M. P., Kao J. P., and Matteson D. R. (2004) Cellular Physiology, pp. 37–52 Elsevier, Philadelphia, PA
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

