Identification and characterization of a direct activator of a gene transfer agent
- PMID: 30723210
- PMCID: PMC6363796
- DOI: 10.1038/s41467-019-08526-1
Identification and characterization of a direct activator of a gene transfer agent
Abstract
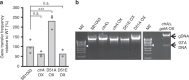
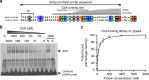
Gene transfer agents (GTAs) are thought to be ancient bacteriophages that have been co-opted into serving their host and can now transfer any gene between bacteria. Production of GTAs is controlled by several global regulators through unclear mechanisms. In Rhodobacter capsulatus, gene rcc01865 encodes a putative regulatory protein that is essential for GTA production. Here, I show that rcc01865 (hereafter gafA) encodes a transcriptional regulator that binds to the GTA promoter to initiate production of structural and DNA packaging components. Expression of gafA is in turn controlled by the pleiotropic regulator protein CtrA and the quorum-sensing regulator GtaR. GafA and CtrA work together to promote GTA maturation and eventual release through cell lysis. Identification of GafA as a direct GTA regulator allows the first integrated regulatory model to be proposed and paves the way for discovery of GTAs in other species that possess gafA homologues.
Conflict of interest statement
The author declares no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

