Cytoskeletal stiffening in synthetic hydrogels
- PMID: 30723211
- PMCID: PMC6363731
- DOI: 10.1038/s41467-019-08569-4
Cytoskeletal stiffening in synthetic hydrogels
Abstract
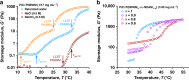
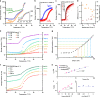
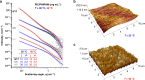
Although common in biology, controlled stiffening of hydrogels in vitro is difficult to achieve; the required stimuli are commonly large and/or the stiffening amplitudes small. Here, we describe the hierarchical mechanics of ultra-responsive hybrid hydrogels composed of two synthetic networks, one semi-flexible and stress-responsive, the other flexible and thermoresponsive. Heating collapses the flexible network, which generates internal stress that causes the hybrid gel to stiffen up to 50 times its original modulus; an effect that is instantaneous and fully reversible. The average generated forces amount to ~1 pN per network fibre, which are similar to values found for stiffening resulting from myosin molecular motors in actin. The excellent control, reversible nature and large response gives access to many biological and bio-like applications, including tissue engineering with truly dynamic mechanics and life-like matter.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Gyles DA, Castro LD, Silva JOC, Ribeiro-Costa RM. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017;88:373–392. doi: 10.1016/j.eurpolymj.2017.01.027. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

