Link between the numbers of particles and variants founding new HIV-1 infections depends on the timing of transmission
- PMID: 30723550
- PMCID: PMC6354028
- DOI: 10.1093/ve/vey038
Link between the numbers of particles and variants founding new HIV-1 infections depends on the timing of transmission
Abstract
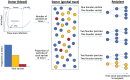
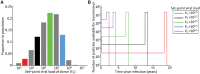
Understanding which HIV-1 variants are most likely to be transmitted is important for vaccine design and predicting virus evolution. Since most infections are founded by single variants, it has been suggested that selection at transmission has a key role in governing which variants are transmitted. We show that the composition of the viral population within the donor at the time of transmission is also important. To support this argument, we developed a probabilistic model describing HIV-1 transmission in an untreated population, and parameterised the model using both within-host next generation sequencing data and population-level epidemiological data on heterosexual transmission. The most basic HIV-1 transmission models cannot explain simultaneously the low probability of transmission and the non-negligible proportion of infections founded by multiple variants. In our model, transmission can only occur when environmental conditions are appropriate (e.g. abrasions are present in the genital tract of the potential recipient), allowing these observations to be reconciled. As well as reproducing features of transmission in real populations, our model demonstrates that, contrary to expectation, there is not a simple link between the number of viral variants and the number of viral particles founding each new infection. These quantities depend on the timing of transmission, and infections can be founded with small numbers of variants yet large numbers of particles. Including selection, or a bias towards early transmission (e.g. due to treatment), acts to enhance this conclusion. In addition, we find that infections initiated by multiple variants are most likely to have derived from donors with intermediate set-point viral loads, and not from individuals with high set-point viral loads as might be expected. We therefore emphasise the importance of considering viral diversity in donors, and the timings of transmissions, when trying to discern the complex factors governing single or multiple variant transmission.
Keywords: HIV-1 vaccine; mathematical modelling; selection bottleneck; transmitted HIV-1 variants; viral evolution; virus transmission.
Figures

References
-
- Baggaley R. F. et al. (2006) ‘Risk of HIV-1 Transmission for Parenteral Exposure and Blood Transfusion: A Systematic Review and Meta-Analysis’, AIDS (London, England), 20: 805–12. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

