Extraordinary centromeres: differences in the meiotic chromosomes of two rock lizards species Darevskia portschinskii and Darevskia raddei
- PMID: 30723630
- PMCID: PMC6359900
- DOI: 10.7717/peerj.6360
Extraordinary centromeres: differences in the meiotic chromosomes of two rock lizards species Darevskia portschinskii and Darevskia raddei
Abstract
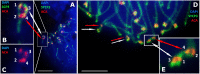
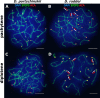
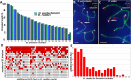
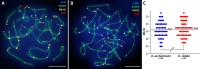
According to the synthesis of 30 years of multidisciplinary studies, parthenogenetic species of rock lizards of genus Darevskia were formed as a result of different combination patterns of interspecific hybridization of the four bisexual parental species: Darevskia raddei, D. mixta, D. valentini, and D. portschinskii. In particular, D. portschinskii and D. raddei are considered as the parental species for the parthenogenetic species D. rostombekowi. Here for the first time, we present the result of comparative immunocytochemical study of primary spermatocyte nuclei spreads from the leptotene to diplotene stages of meiotic prophase I in two species: D. portschinskii and D. raddei. We observed similar chromosome lengths for both synaptonemal complex (SC) karyotypes as well as a similar number of crossing over sites. However, unexpected differences in the number and distribution of anti-centromere antibody (ACA) foci were detected in the SC structure of bivalents of the two species. In all examined D. portschinskii spermatocyte nuclei, one immunostained centromere focus was detected per SC bivalent. In contrast, in almost every studied D. raddei nuclei we identified three to nine SCs with additional immunostained ACA foci per SC bivalent. Thus, the obtained results allow us to identify species-specific karyotype features, previously not been detected using conventional mitotic chromosome analysis. Presumably the additional centromere foci are result of epigenetic chromatin modifications. We assume that this characteristic of the D. raddei karyotype could represent useful marker for the future studies of parthenogenetic species hybrid karyotypes related to D. raddei.
Keywords: Darevskia lizards; Dicentric chromosomes; Meiosis; Neocentromere; Reticulate evolution; Synaptonemal complex.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Bogdanov YF, Kolomiets OL. Synaptonemal Complex as an Indicator of the Dynamics of Meiosis and Chromosome Variation. Moscow: KMK; 2007. p. 358. [in Russian]
-
- Borkin LJ, Darevsky IS. Reticular (hybridogeneous) speciation in vertebrate. Zhurnal Obshchei Biologii. 1980;41:485–506. [in Russian]
Associated data
LinkOut - more resources
Full Text Sources

