A phase 1 clinical trial of the sigma-2 receptor complex allosteric antagonist CT1812, a novel therapeutic candidate for Alzheimer's disease
- PMID: 30723776
- PMCID: PMC6352291
- DOI: 10.1016/j.trci.2018.11.001
A phase 1 clinical trial of the sigma-2 receptor complex allosteric antagonist CT1812, a novel therapeutic candidate for Alzheimer's disease
Abstract
Background: Elayta (CT1812) is a novel allosteric antagonist of the sigma-2 receptor complex that prevents and displaces binding of Aβ oligomers to neurons. By stopping a key initiating event in Alzheimer's disease, this first-in-class drug candidate mitigates downstream synaptotoxicity and restores cognitive function in aged transgenic mouse models of Alzheimer's disease.
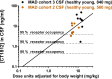
Methods: A phase 1, two-part single and multiple ascending dose study was conducted in 7 and 4 cohorts of healthy human subjects, respectively. In part A, healthy, young subjects (<65 years old) received CT1812 doses ranging from 10 to 1120 mg (6:2 active to placebo [A:P] per cohort). In part B, subjects were administered 280, 560, and 840 mg once daily for 14 days (8:2 A:P per cohort). An elderly cohort, aged 65-75 years, was dosed at 560 mg once daily for 14 days (7:2 A:P). Serum concentrations of CT1812 in part B were measured on day 3 and 14 and cerebrospinal fluid concentrations on day 7 or 9. Cognitive testing was performed in the healthy elderly cohort at baseline and at day 14 of treatment.
Results: Treatment with CT1812 was well tolerated in all cohorts. Adverse events were mild to moderate in severity and included headache and GI tract symptoms. Plasma concentrations of drug were dose proportional across two orders of magnitude with minimal accumulation over 14 days. Cognitive scores in the healthy elderly cohort were similar before and after treatment.
Conclusions: CT1812 was well tolerated with single dose administration up to 1120 mg and with multiple dose administration up to 840 mg and 560 mg in healthy young and healthy elderly subjects, respectively. CT1812 is currently being studied in early phase 2 trials in patients with Alzheimer's disease.
Keywords: Alzheimer's disease (AD); Amyloid beta (Aβ); CT1812; Cerebrospinal fluid (CSF); Clinical trial; Multiple ascending dose (MAD); Pharmacokinetics; Safety; Single ascending dose (SAD); Therapy.
Figures

References
-
- 2018 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2018;14:367–429.
-
- Hebert L.E., Beckett L.A., Scherr P.A., Evans D.A. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord. 2001;15:169–173. - PubMed
-
- Murphy S.L., Xu J., Kochanek K.D., Curtin S.C., Arias E. National Vital Statistics Reports Volume 66, Number 6, November 27, 2017. https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_06.pdf Available at:
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
