Phase I dose-escalation study of capmatinib (INC280) in Japanese patients with advanced solid tumors
- PMID: 30724423
- PMCID: PMC6447844
- DOI: 10.1111/cas.13956
Phase I dose-escalation study of capmatinib (INC280) in Japanese patients with advanced solid tumors
Abstract
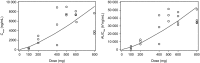
Capmatinib is a highly specific, potent and selective MET inhibitor. This was an open-label, multicenter, dose-escalation, phase I study conducted in Japanese patients with advanced solid tumors (not selected based on their MET status). The primary objective was to determine the maximum tolerated dose (MTD) and/or highest studied dose being safe. Secondary objectives included safety, pharmacokinetics and preliminary antitumor activity. Dose escalation was guided by a Bayesian Logistic Regression Model dependent on dose-limiting toxicities (DLT) in cycle 1. Of 44 adult Japanese patients with confirmed advanced solid tumors enrolled, 29 received capmatinib capsules (doses ranging from 100 mg once daily [q.d.] to 600 mg twice daily [b.i.d.]) and 15 received tablets (200 mg b.i.d. and 400 mg b.i.d.). DLT occurred in two patients: grade 2 suicidal ideation (600 mg b.i.d. capsule) and grade 3 depression (400 mg b.i.d. tablet). MTD was not reached. The highest studied dose determined to be safe as tablet was 400 mg b.i.d., whereas it is not yet determined for capsules. Most common adverse events suspected to be drug-related were increased blood creatinine, nausea, decreased appetite, vomiting and diarrhea. Following repeated daily dosing up to day 15 by q.d. or b.i.d. regimen using capsules, median time to reach maximum plasma drug concentration (Tmax ) was 1.0-4.0 hours; absorption was more rapid after dosing using tablets, with median Tmax of 1.0 hour on both days 1 and 15. Eight patients had a best overall response of stable disease. These data support further clinical development of capmatinib.
Keywords: MET; INC280; Japan; capmatinib; phase 1.
© 2019 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Annual value of remuneration received: Kae Ishihara from Novartis Pharma K.K. (employment); Tomoyuki Kakizume from Novartis Pharma K.K. (employment); Kazuto Natsume from Novartis Pharma K.K. (employment); and Andrea Myers from Novartis Institute for Biomedical Research (employment). Annual profit from shares received: Andrea Myers has Novartis stock. Total annual value of daily allowances/honoraria received: Taito Esaki from Eli Lilly; Yoichi Naito from Chugai, Novartis and Pfizer; and Kiyotaka Yoh from Chugai, Eli Lilly and Astra Zeneca. Total annual value of research funds, endowments, endowed chairs and researcher‐employment costs received: Taito Esaki from Eli Lilly, Daiichi‐Sankyo, Merck Serono, Taiho, MSD, Novartis, Dainippon Sumitomo, Ono and Boehringer; Takashi Seto from Novartis Pharma; Hideaki Bando from Astra Zeneca and Sysmex; Yoichi Naito from Astra Zeneca, Pfizer and Eli Lilly; Kiyotaka Yoh from Bayer, Pfizer, Eli Lilly, Astra Zeneca, Taiho, Novartis and MSD; and Toshihiko Doi from Novartis. Total annual value of scholarship (incentive) endowments or research grants received: Taito Esaki from Ono.
Figures


References
-
- Birchmeier C, Birchmeier W, Gherardi E, et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915‐925. - PubMed
-
- Christensen JG, Burrows J, Salgia R, et al. c‐MET as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett. 2005;225:1340‐26. - PubMed
-
- Lal B, Xia S, Abounader R, et al. Targeting the c‐Met pathway potentiates glioblastoma responses to gamma‐radiation. Clin Cancer Res. 2005;11:4479‐4486. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

