Preliminary Assignment of Protonated and Deprotonated Homocitrates in Extracted FeMo-Cofactors by Comparisons with Molybdenum(IV) Lactates and Oxidovanadium Glycolates
- PMID: 30726074
- PMCID: PMC6813765
- DOI: 10.1021/acs.inorgchem.8b03108
Preliminary Assignment of Protonated and Deprotonated Homocitrates in Extracted FeMo-Cofactors by Comparisons with Molybdenum(IV) Lactates and Oxidovanadium Glycolates
Abstract
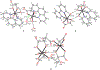
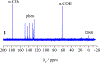
A similar pair of protonated and deprotonated mononuclear oxidovanadium glycolates [VO(Hglyc)(phen)(H2O)]Cl·2H2O (1) and [VO(glyc)(bpy)(H2O)] (2) and a mixed-(de)protonated oxidovanadium triglycolate (NH4)2[VO(Hglyc)2(glyc)]·H2O (3) were isolated and examined. The ≡C-O(H) (≡C-OH or ≡C-O) groups coordinated to vanadium were spectroscopically and structurally identified. The glycolate in 1 features a bidentate chelation through protonated α-hydroxy and α-carboxy groups, whereas the glycolate in 2 coordinates through deprotonated α-alkoxy and α-carboxy groups. The glycolates in 3 coordinate to vanadium through α-alkoxy or α-hydroxy and α-carboxy groups and thus have both protonated ≡C-OH and deprotonated ≡C-O bonds simultaneously. Structural investigations revealed that the longer protonated V-Oα-hydroxy bonds [2.234(2) Å and 2.244(2) Å] in 1 and 3 are close to those of FeV-cofactor (FeV-co) 2.17 Å1 (FeMo-co 2.17 Å2), while deprotonated V-Oα-alkoxy bonds [2, 1.930(2); 3, 1.927(2) Å] were obviously shorter. This shows a similar elongated trend as the Mo-O distances in the previously reported deprotonated vs protonated molybdenum lactates (Wang, S. Y. et al. Dalton Trans. 2018, 47, 7412-7421) and these vanadium and molybdenum complexes have the same local V/Mo-homocitrate structures as those of FeV/Mo-cos of nitrogenases. The IR spectra of these oxidovanadium and the previously synthesized molybdenum complexes including different substituted ≡C-O(H) model compounds show red-shifts for ≡C-OH vs ≡C-O alternation, which further assign the two IR bands of extracted FeMo-co at 1084 and 1031 cm-1 to ≡C-O and ≡C-OH vibrations, respectively. Although the structural data or IR spectra for some of the previously synthesized Mo/V complexes and extracted FeMo-co were measured earlier, this is the first time that the ≡C-O(H) coordinated peaks are assigned. The overall structural and IR results well suggest the coexistence of homocitrates coordinated with α-alkoxy (deprotonated) and α-hydroxy (protonated) groups in the extracted FeMo-co.
Conflict of interest statement
Wang-Tin Jin and Hongxin Wang are co-first authors. The authors declare no competing financial interest.
Figures
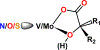


References
-
- Wang SY; Jin WT; Chen HB; Zhou ZH Comparison of hydroxycarboxylato imidazole molybdenum(iv) complexes and nitrogenase protein structures: indirect evidence for the protonation of homocitrato FeMo-cofactors, Dalton Trans 2018, 47, 7412–7421. - PubMed
-
- Einsle O; Tezcan FA; Andrade SL; Schmid B; Yoshida M; Howard JB; Rees DC Nitrogenase MoFe-protein at 1.16 Å resolution: a central ligand in the FeMo-cofactor, Science 2002, 297, 1696–1700. - PubMed

