Muscle cannabinoid 1 receptor regulates Il-6 and myostatin expression, governing physical performance and whole-body metabolism
- PMID: 30726112
- PMCID: PMC6988864
- DOI: 10.1096/fj.201801145R
Muscle cannabinoid 1 receptor regulates Il-6 and myostatin expression, governing physical performance and whole-body metabolism
Abstract
Sarcopenic obesity, the combination of skeletal muscle mass and function loss with an increase in body fat, is associated with physical limitations, cardiovascular diseases, metabolic stress, and increased risk of mortality. Cannabinoid receptor type 1 (CB1R) plays a critical role in the regulation of whole-body energy metabolism because of its involvement in controlling appetite, fuel distribution, and utilization. Inhibition of CB1R improves insulin secretion and insulin sensitivity in pancreatic β-cells and hepatocytes. We have now developed a skeletal muscle-specific CB1R-knockout (Skm-CB1R-/-) mouse to study the specific role of CB1R in muscle. Muscle-CB1R ablation prevented diet-induced and age-induced insulin resistance by increasing IR signaling. Moreover, muscle-CB1R ablation enhanced AKT signaling, reducing myostatin expression and increasing IL-6 secretion. Subsequently, muscle-CB1R ablation increased myogenesis through its action on MAPK-mediated myogenic gene expression. Consequently, Skm-CB1R-/- mice had increased muscle mass and whole-body lean/fat ratio in obesity and aging. Muscle-CB1R ablation improved mitochondrial performance, leading to increased whole-body muscle energy expenditure and improved physical endurance, with no change in body weight. These results collectively show that CB1R in muscle is sufficient to regulate whole-body metabolism and physical performance and is a novel target for the treatment of sarcopenic obesity. -González-Mariscal, I., Montoro, R. A., O'Connell, J. F., Kim, Y., Gonzalez-Freire, M., Liu, Q.-R., Alfaras, I., Carlson, O. D., Lehrmann, E., Zhang, Y., Becker, K. G., Hardivillé, S., Ghosh, P., Egan, J. M. Muscle cannabinoid 1 receptor regulates Il-6 and myostatin expression, governing physical performance and whole-body metabolism.
Keywords: CB1R; insulin sensitivity; myokines; skeletal muscle.
Conflict of interest statement
This work was supported by the U.S. National Institutes of Health, National Institute on Aging Intramural Research Program. S961 was donated by Novo Nordisk. The authors declare no conflicts of interest.
Figures
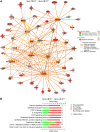

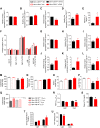

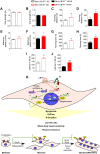
References
-
- Waters D. L., Baumgartner R. N. (2011) Sarcopenia and obesity. Clin. Geriatr. Med. 27, 401–421 - PubMed
-
- Pedersen B. K., Febbraio M. A. (2012) Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465 - PubMed
-
- Langley B., Thomas M., Bishop A., Sharma M., Gilmour S., Kambadur R. (2002) Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J. Biol. Chem. 277, 49831–49840 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

