Durable Tumor Regression and Overall Survival in Patients With Advanced Merkel Cell Carcinoma Receiving Pembrolizumab as First-Line Therapy
- PMID: 30726175
- PMCID: PMC6424137
- DOI: 10.1200/JCO.18.01896
Durable Tumor Regression and Overall Survival in Patients With Advanced Merkel Cell Carcinoma Receiving Pembrolizumab as First-Line Therapy
Abstract
Purpose: Merkel cell carcinoma (MCC) is an aggressive skin cancer often caused by the Merkel cell polyomavirus. Clinical trials of programmed cell death-1 pathway inhibitors for advanced MCC (aMCC) demonstrate increased progression-free survival (PFS) compared with historical chemotherapy data. However, response durability and overall survival (OS) data are limited.
Patients and methods: In this multicenter phase II trial (Cancer Immunotherapy Trials Network-09/Keynote-017), 50 adults naïve to systemic therapy for aMCC received pembrolizumab (2 mg/kg every 3 weeks) for up to 2 years. Radiographic responses were assessed centrally per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1.
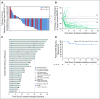
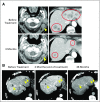
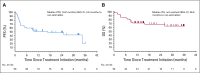
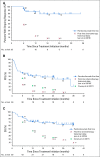
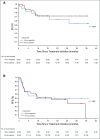
Results: Among 50 patients, the median age was 70.5 years, and 64% had Merkel cell polyomavirus-positive tumors. The objective response rate (ORR) to pembrolizumab was 56% (complete response [24%] plus partial response [32%]; 95% CI, 41.3% to 70.0%), with ORRs of 59% in virus-positive and 53% in virus-negative tumors. Median follow-up time was 14.9 months (range, 0.4 to 36.4+ months). Among 28 responders, median response duration was not reached (range, 5.9 to 34.5+ months). The 24-month PFS rate was 48.3%, and median PFS time was 16.8 months (95% CI, 4.6 months to not estimable). The 24-month OS rate was 68.7%, and median OS time was not reached. Although tumor viral status did not correlate with ORR, PFS, or OS, there was a trend toward improved PFS and OS in patients with programmed death ligand-1-positive tumors. Grade 3 or greater treatment-related adverse events occurred in 14 (28%) of 50 patients and led to treatment discontinuation in seven (14%) of 50 patients, including one treatment-related death.
Conclusion: Here, we present the longest observation to date of patients with aMCC receiving first-line anti-programmed cell death-1 therapy. Pembrolizumab demonstrated durable tumor control, a generally manageable safety profile, and favorable OS compared with historical data from patients treated with first-line chemotherapy.
Trial registration: ClinicalTrials.gov NCT02267603.
Figures

References
-
- Wong SQ, Waldeck K, Vergara IA, et al. UV-associated mutations underlie the etiology of MCV-negative Merkel cell carcinomas. Cancer Res. 2015;75:5228–5234. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

