Synchronization of pancreatic islets by periodic or non-periodic muscarinic agonist pulse trains
- PMID: 30726280
- PMCID: PMC6364940
- DOI: 10.1371/journal.pone.0211832
Synchronization of pancreatic islets by periodic or non-periodic muscarinic agonist pulse trains
Abstract
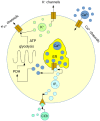
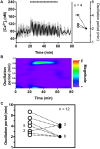
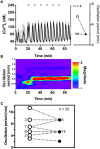
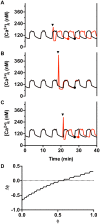
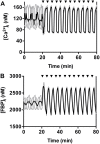
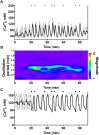
Pulsatile insulin secretion into the portal vein from the many pancreatic islets of Langerhans is critical for efficient glucose homeostasis. The islets are themselves endogenous oscillators, but since they are not physically coupled it is not obvious how their oscillations are synchronized across the pancreas. It has been proposed that synchronization of islets is achieved through periodic activity of intrapancreatic ganglia, and indeed there are data supporting this proposal. Postganglionic nerves are cholinergic, and their product, acetylcholine, can influence islet β-cells through actions on M3 muscarinic receptors which are coupled to Gq type G-proteins. In addition, the neurons secrete several peptide hormones that act on β-cell receptors. The data supporting synchronization via intrapancreatic ganglia are, however, limited. In particular, it has not been shown that trains of muscarinic pulses are effective at synchronizing islets in vitro. Also, if as has been suggested, there is a ganglionic pacemaker driving islets to a preferred frequency, no neural circuitry for this pacemaker has been identified. In this study, both points are addressed using a microfluidic system that allows for the pulsed application of the muscarinic agonist carbachol. We find that murine islets are entrained and synchronized over a wide range of frequencies when the carbachol pulsing is periodic, adding support to the hypothesis that ganglia can synchronize islets in vivo. We also find that islet synchronization is very effective even if the carbachol pulses are applied at random times. This suggests that a neural pacemaker is not needed; all that is required is that islets receive occasional coordinated input from postganglionic neurons. The endogenous rhythmic activity of the islets then sets the frequency of the islet population rhythm, while the input from ganglia acts only to keep the islet oscillators in phase.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

