Low Sodium Diet Decreases Stone Formation in Genetic Hypercalciuric Stone-Forming Rats
- PMID: 30726853
- PMCID: PMC6546529
- DOI: 10.1159/000497117
Low Sodium Diet Decreases Stone Formation in Genetic Hypercalciuric Stone-Forming Rats
Abstract
Background: Urine (u) calcium (Ca) excretion is directly dependent on dietary sodium (Na) intake leading to the recommendation for Na restriction in hypercalciuric kidney stone formers. However, there is no direct evidence that limiting Na intake will reduce recurrent stone formation.
Materials and methods: We used genetic hypercalciuric stone-forming (GHS) rats, which universally form Ca phosphate (P) kidney stones, fed either a low Na (LNa, 0.05%) or normal Na (NNa, 0.4%) Na diet (D) for 18 weeks. Urine was collected at 6-week intervals. Radiographic analysis for stone formation and bone analyses were done at the conclusion of the study.
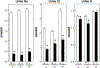
Results: Mean uCa was lower with LNaD than NNaD as was uP and LNaD decreased mean uNa and uChloride. There were no differences in urine supersaturation (SS) with respect to calcium phosphate (CaP) or Ca oxalate (CaOx). However, stone formation was markedly decreased with LNaD by radiographic analysis. The LNaD group had significantly lower femoral anterior-posterior diameter and volumetric bone mineral density (vBMD), but no change in vertebral trabecular vBMD. There were no differences in the bone formation rate or osteoclastic bone resorption between groups. The LNaD group had significantly lower femoral stiffness; however, the ultimate load and energy to fail was not different.
Conclusion: Thus, a low Na diet reduced uCa and stone formation in GHS rats, even though SS with respect to CaP and CaOx was unchanged and effects on bone were modest. These data, if confirmed in humans, support dietary Na restriction to prevent recurrent Ca nephrolithiasis.
Keywords: Mineral metabolism; Nephrolithiasis; Sodium.
© 2019 S. Karger AG, Basel.
Conflict of interest statement
DISCLOSURE STATEMENT
The authors have no competing financial interests and nothing to disclose.
Figures







Comment in
-
Re: Low Sodium Diet Decreases Stone Formation in Genetic Hypercalciuric Stone-Forming Rats.J Urol. 2019 Oct;202(4):656. doi: 10.1097/01.JU.0000577244.55221.c8. Epub 2019 Sep 6. J Urol. 2019. PMID: 31294665 No abstract available.
References
-
- Bushinsky DA, Coe FL, Moe OW: Nephrolithiasis; in Brenner BM (ed): The Kidney. Philadelphia, W.B. Saunders, 2012, vol 2, pp 1455–1507.
-
- Bose A, Monk RD, Bushinsky DA: Kidney stones; in Melmed S, Polonsky KS, Larsen PR, Kronenberg HM (eds): Williams Textbook of Endocrinology. Philadelphia, Elsevier, 2016, pp 1365–1384.
-
- Stechman MJ, Loh NY, Thakker RV: Genetics of hypercalciuric nephrolithiasis: renal stone disease. Ann NY Acad Sci 2007;1116:461–484. - PubMed
-
- Moe OW, Bushinsky DA: Genetic Hypercalciuria: A Major Risk Factor in Kidney Stones; in Thakker RV, Whyte MP, Eisman JA, Igarashi T (eds): Genetics of Bone Biology and Skeletal Disease. London, UK, Elsevier, 2013, pp 585–604.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

