Promoters to Study Vascular Smooth Muscle
- PMID: 30727757
- PMCID: PMC6527360
- DOI: 10.1161/ATVBAHA.119.312449
Promoters to Study Vascular Smooth Muscle
Abstract
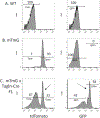
Smooth muscle cells (SMCs) are a critical component of blood vessel walls that provide structural support, regulate vascular tone, and allow for vascular remodeling. These cells also exhibit a remarkable plasticity that contributes to vascular growth and repair but also to cardiovascular pathologies, including atherosclerosis, intimal hyperplasia and restenosis, aneurysm, and transplant vasculopathy. Mouse models have been an important tool for the study of SMC functions. The development of smooth muscle-expressing Cre-driver lines has allowed for exciting discoveries, including recent advances revealing the diversity of phenotypes derived from mature SMC transdifferentiation in vivo using inducible CreER T2 lines. We review SMC-targeting Cre lines driven by the Myh11, Tagln, and Acta2 promoters, including important technical considerations associated with these models. Limitations that can complicate study of the vasculature include expression in visceral SMCs leading to confounding phenotypes, and expression in multiple nonsmooth muscle cell types, such as Acta2-Cre expression in myofibroblasts. Notably, the frequently employed Tagln/ SM22α- Cre driver expresses in the embryonic heart but can also confer expression in nonmuscular cells including perivascular adipocytes and their precursors, myeloid cells, and platelets, with important implications for interpretation of cardiovascular phenotypes. With new Cre-driver lines under development and the increasing use of fate mapping methods, we are entering an exciting new era in SMC research.
Keywords: atherosclerosis; gene expression; mice, transgenic; muscle, smooth; myeloid cells; neointima.
Figures

References
-
- Lee RM, Owens GK, Scott-Burden T, Head RJ, Mulvany MJ and Schiffrin EL. Pathophysiology of smooth muscle in hypertension. Canadian journal of physiology and pharmacology 1995;73:574–84. - PubMed
-
- Alexander MR and Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol 2012;74:13–40. - PubMed
-
- Sauer B and Henderson N. The cyclization of linear DNA in Escherichia coli by site-specific recombination. Gene 1988;70:331–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

