Romidepsin suppresses monosodium urate crystal-induced cytokine production through upregulation of suppressor of cytokine signaling 1 expression
- PMID: 30728075
- PMCID: PMC6366029
- DOI: 10.1186/s13075-019-1834-x
Romidepsin suppresses monosodium urate crystal-induced cytokine production through upregulation of suppressor of cytokine signaling 1 expression
Abstract
Background: Acute gouty arthritis currently is the most common form of inflammatory arthritis in developed countries. Treatment is still suboptimal. Dosage of urate-lowering therapy is often too low to reach target urate levels, and adherence to therapy is poor. In this study, we therefore explore a new treatment option to limit inflammation in acute gout: specific histone deacetylase (HDAC) inhibition.
Methods: Peripheral blood mononuclear cells (PBMCs) were cultured with a combination of monosodium urate crystals (MSU) and palmitic acid (C16.0) in order to activate the NLRP3 inflammasome and induce IL-1β production. HDAC inhibitors and other compounds were added beforehand with a 1-h pre-incubation period.
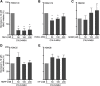
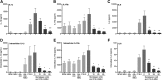
Results: The HDAC1/2 inhibitor romidepsin was most potent in lowering C16.0+MSU-induced IL-1β production compared to other specific class I HDAC inhibitors. At 10 nM, romidepsin decreased IL-1β, IL-1Ra, IL-6, and IL-8 production. IL-1β mRNA was significantly decreased at 25 nM. Although romidepsin increased PTEN expression, PBMCs from patients with germline mutations in PTEN still responded well to romidepsin. Romidepsin also increased SOCS1 expression and blocked STAT1 and STAT3 activation. Furthermore, experiments with bortezomib showed that blocking the proteasome reverses the cytokine suppression by romidepsin.
Conclusions: Our results show that romidepsin is a very potent inhibitor of C16.0+MSU-induced cytokines in vitro. Romidepsin upregulated transcription of SOCS1, which was shown to directly target inflammatory signaling molecules for proteasomal degradation. Inhibiting the proteasome therefore reversed the cytokine-suppressive effects of romidepsin. HDAC1/2 dual inhibition could therefore be a highly potent new treatment option for acute gout, although safety has to be determined in vivo.
Keywords: Cytokines; Gout; HDAC; Inflammation.
Conflict of interest statement
Ethics approval and consent to participate
Buffy coats from healthy donors were obtained after written informed consent from Sanquin Blood Bank, Nijmegen, the Netherlands. Blood collection from Cowden syndrome patients was approved by the accredited medical research and ethics committee of the region Arnhem/Nijmegen in the Netherlands (reference 2014/147). Written informed consent was obtained before inclusion. All experiments with human material were performed according to the declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Kiadaliri AA, Uhlig T, Englund M. Burden of gout in the Nordic region, 1990-2015: findings from the Global Burden of Disease Study 2015. Scand J Rheumatol. 2018;47(5):410–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 12-02-303/Reumafonds/International
- MSc-PhD grant/Radboud Universitair Medisch Centrum/International
- Spinoza grant 2016/Nederlandse Organisatie voor Wetenschappelijk Onderzoek/International
- 310372/ERC_/European Research Council/International
- P_37_762, MySMIS 103587/Romanian Ministry of European Funds/International
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

