Clinical characteristics and outcomes of immune checkpoint inhibitor-induced pancreatic injury
- PMID: 30728076
- PMCID: PMC6364483
- DOI: 10.1186/s40425-019-0502-7
Clinical characteristics and outcomes of immune checkpoint inhibitor-induced pancreatic injury
Abstract
Background: Immune checkpoint inhibitor (ICI)-induced pancreatic injury (ICIPI) is not well documented in the literature. We aimed to describe the clinical characteristics and outcomes of patients who developed ICIPI.
Methods: We reviewed the medical records of consecutive patients who had a confirmed diagnosis of ICIPI (Common Terminology Criteria for Adverse Events grade ≥ 3 lipase elevation with or without clinical symptoms) from April 2011 through April 2018.
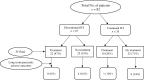
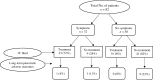
Results: Among the 2,279 patients received ICI and had lipase values checked thereafter, 82 (4%) developed ICIPI. Overall, 65% of patients received inhibitors of programmed death protein-1 or its ligand. Compared with asymptomatic presentation, patients who had clinical symptoms of pancreatitis (n = 32) had higher levels of lipase (P = 0.032), more frequent imaging evidence of pancreatitis (P = 0.055), and more frequent hospitalization (P < 0.001) and received intravenous fluids (P < 0.001) and steroids more frequently (P = 0.008). Twelve patients (15%) developed long-term adverse outcomes of ICIPI; three had chronic pancreatitis, four had recurrence of ICIPI, and six had subsequent diabetes. Among 35 patients who resumed ICI therapy, four (11%) had recurrence of lipase elevation. Logistic regression revealed that smoking and hyperlipidemia were associated with increased risk for long-term adverse outcomes of ICIPI, and intravenous fluids were associated with reduced risk. Patients who resumed ICI therapy survived longer than patients who discontinued ICI therapy permanently, statistically not significant (P = 0.0559). Patients who developed long-term adverse outcomes of ICIPI survived significantly longer than those who did not (P = 0.0295). The highest proportion of patients (6/21, 29%) developed long-term adverse outcomes of ICIPI was among those without typical symptoms of pancreatitis, continued ICI therapy after ICIPI, and did not receive intravenous fluids.
Conclusion: ICIPI can present as typical acute pancreatitis, with risk of the development of a pseudocyst, diabetes, and chronic pancreatitis. ICI resumption after ICIPI may lead to recurrence of lipase elevation without increased risk of long-term adverse outcomes, and can increase survival duration. Intravenous fluids may prevent long-term adverse outcomes, but steroids do not appear to affect outcomes of ICIPI. Asymptomatic ICIPI presentation may lead to undertreatment of ICIPI owing to underestimation of its degree, and therefore, intravenous fluid administration could potentially could potentially be benificial to prevent long-term adverse outcomes even in asymptomatic patients.
Keywords: Cytotoxic T-cell lymphocyte-4 (CTLA-4); Immune checkpoint inhibitor; Immunotherapy; Lipase elevation; Pancreatic adverse events; Pancreatic injury; Pancreatitis; Programmed death-1 and ligand-1 (PD-1/L1).
Conflict of interest statement
Ethics approval and consent to participate
This retrospective, single-center study was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center.
Consent for publication
This study was granted waiver for consent.
Competing interests
Dr. Amir A Jazaeri received research funding from Iovance, AstraZeneca, Bristol-Myers Squibb, Pfizer, Lilly, served on Advisory Board for Iovance, Aravive, Almac, and Data Safety Monitoring Board for Genetech. Dr. Ramona Dadu received research funding from Merck, Eisai, AstraZeneca, and served on Advisory Board for Bristol-Myers Squibb. The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Kaufman Howard L., Russell Jeffery, Hamid Omid, Bhatia Shailender, Terheyden Patrick, D'Angelo Sandra P., et al., Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol, 2016. 17(10): p. 1374–1385. - PMC - PubMed
-
- Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, et al. Safety and efficacy of Durvalumab (MEDI4736), an anti-programmed cell death Ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder Cancer. J Clin Oncol. 2016;34(26):3119–3125. doi: 10.1200/JCO.2016.67.9761. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous