Eosinophil-derived IL-13 promotes emphysema
- PMID: 30728205
- PMCID: PMC7313423
- DOI: 10.1183/13993003.01291-2018
Eosinophil-derived IL-13 promotes emphysema
Abstract
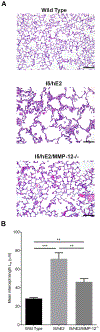
The inflammatory responses in chronic airway diseases leading to emphysema are not fully defined. We hypothesised that lung eosinophilia contributes to airspace enlargement in a mouse model and to emphysema in patients with chronic obstructive pulmonary disease (COPD).A transgenic mouse model of chronic type 2 pulmonary inflammation (I5/hE2) was used to examine eosinophil-dependent mechanisms leading to airspace enlargement. Human sputum samples were collected for translational studies examining eosinophilia and matrix metalloprotease (MMP)-12 levels in patients with chronic airways disease.Airspace enlargement was identified in I5/hE2 mice and was dependent on eosinophils. Examination of I5/hE2 bronchoalveolar lavage identified elevated MMP-12, a mediator of emphysema. We showed, in vitro, that eosinophil-derived interleukin (IL)-13 promoted alveolar macrophage MMP-12 production. Airspace enlargement in I5/hE2 mice was dependent on MMP-12 and eosinophil-derived IL-4/13. Consistent with this, MMP-12 was elevated in patients with sputum eosinophilia and computed tomography evidence of emphysema, and also negatively correlated with forced expiratory volume in 1 s.A mouse model of chronic type 2 pulmonary inflammation exhibited airspace enlargement dependent on MMP-12 and eosinophil-derived IL-4/13. In chronic airways disease patients, lung eosinophilia was associated with elevated MMP-12 levels, which was a predictor of emphysema. These findings suggest an underappreciated mechanism by which eosinophils contribute to the pathologies associated with asthma and COPD.
Copyright ©ERS 2019.
Conflict of interest statement
Conflict of interest: A.D. Doyle reports grants from NIH (F31HL124959), donor money paid to the Mayo Clinic from Donald R. Levin Family Foundation, and a scholarship at the Mayo Clinic from Mayo Clinic Sidney Luckman Family Predoctoral Fellowship, during the conduct of the study. Conflict of interest: M. Mukherjee has nothing to disclose. Conflict of interest: W.E. LeSuer has nothing to disclose. Conflict of interest: T.B. Bittner has nothing to disclose. Conflict of interest: S.M. Pasha has nothing to disclose. Conflict of interest: J.J. Frere has nothing to disclose. Conflict of interest: J.L. Neely has nothing to disclose. Conflict of interest: J.A. Kloeber has nothing to disclose. Conflict of interest: K.P. Shim has nothing to disclose. Conflict of interest: S.I. Ochkur has nothing to disclose. Conflict of interest: T. Ho has nothing to disclose. Conflict of interest: S. Svenningsen has nothing to disclose. Conflict of interest: B.L. Wright reports donor money paid to the Mayo Clinic from Donald R. Levin Family Foundation, during the conduct of the study. Conflict of interest: M.A. Rank has nothing to disclose. Conflict of interest: J.J. Lee received grants from NIH (HL058723), NIH NCRR (K26 RR0109709) and the Mayo Foundation for Medical Education and Research, during the conduct of this study; J.J. Lee is deceased and this statement was made on behalf of the author by the corresponding author. Conflict of interest: P. Nair reports grants and personal fees for consultancy and lecturing from AstraZeneca and Teva, grants from Boehringer Ingelheim, grants and personal fees for lecturing from Novartis, grants and personal fees for consultancy from Sanofi and GSK, and personal fees for consultancy from Theravance and Knopp, outside the submitted work. Conflict of interest: E.A. Jacobsen reports grants from NIH (HL065228), and donor money paid to the Mayo Clinic from Donald R. Levin Family Foundation, during the conduct of the study.
Figures




Comment in
-
It needs more than just eosinophils to cause emphysema in COPD.Eur Respir J. 2019 May 30;53(5):1900332. doi: 10.1183/13993003.00332-2019. Print 2019 May. Eur Respir J. 2019. PMID: 31147422 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
