A multiscale model of mechanotransduction by the ankyrin chains of the NOMPC channel
- PMID: 30728217
- PMCID: PMC6400526
- DOI: 10.1085/jgp.201812266
A multiscale model of mechanotransduction by the ankyrin chains of the NOMPC channel
Abstract
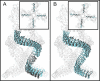
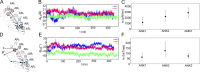
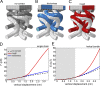
Our senses of touch and hearing are dependent on the conversion of external mechanical forces into electrical impulses by the opening of mechanosensitive channels in sensory cells. This remarkable feat involves the conversion of a macroscopic mechanical displacement into a subnanoscopic conformational change within the ion channel. The mechanosensitive channel NOMPC, responsible for hearing and touch in flies, is a homotetramer composed of four pore-forming transmembrane domains and four helical chains of 29 ankyrin repeats that extend 150 Å into the cytoplasm. Previous work has shown that the ankyrin chains behave as biological springs under extension and that tethering them to microtubules could be involved in the transmission of external forces to the NOMPC gate. Here we combine normal mode analysis (NMA), full-atom molecular dynamics simulations, and continuum mechanics to characterize the material properties of the chains under extreme compression and extension. NMA reveals that the lowest-frequency modes of motion correspond to fourfold symmetric compression/extension along the channel, and the lowest-frequency symmetric mode for the isolated channel domain involves rotations of the TRP domain, a putative gating element. Finite element modeling reveals that the ankyrin chains behave as a soft spring with a linear, effective spring constantof 22 pN/nm for deflections ≤15 Å. Force-balance analysis shows that the entire channel undergoes rigid body rotation during compression, and more importantly, each chain exerts a positive twisting moment on its respective linker helices and TRP domain. This torque is a model-independent consequence of the bundle geometry and would cause a clockwise rotation of the TRP domain when viewed from the cytoplasm. Force transmission to the channel for compressions >15 Å depends on the nature of helix-helix contact. Our work reveals that compression of the ankyrin chains imparts a rotational torque on the TRP domain, which potentially results in channel opening.
© 2019 Argudo et al.
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

