Celestial navigation in Drosophila
- PMID: 30728228
- PMCID: PMC7375828
- DOI: 10.1242/jeb.186148
Celestial navigation in Drosophila
Abstract
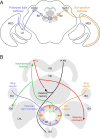
Many casual observers typecast Drosophila melanogaster as a stationary pest that lurks around fruit and wine. However, the omnipresent fruit fly, which thrives even in desert habitats, likely established and maintained its cosmopolitan status via migration over large spatial scales. To perform long-distance dispersal, flies must actively maintain a straight compass heading through the use of external orientation cues, such as those derived from the sky. In this Review, we address how D. melanogaster accomplishes long-distance navigation using celestial cues. We focus on behavioral and physiological studies indicating that fruit flies can navigate both to a pattern of linearly polarized light and to the position of the sun - the same cues utilized by more heralded insect navigators such as monarch butterflies and desert ants. In both cases, fruit flies perform menotaxis, selecting seemingly arbitrary headings that they then maintain over time. We discuss how the fly's nervous system detects and processes this sensory information to direct the steering maneuvers that underlie navigation. In particular, we highlight recent findings that compass neurons in the central complex, a set of midline neuropils, are essential for navigation. Taken together, these results suggest that fruit flies share an ancient, latent capacity for celestial navigation with other insects. Furthermore, they illustrate the potential of D. melanogaster to help us to elucidate both the cellular basis of navigation and mechanisms of directed dispersal on a landscape scale.
Keywords: Central complex; Dispersal; Insects; Migration; Polarized light; Sun compass.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

 /
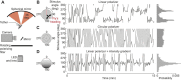
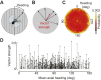
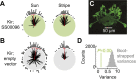
/ ) of Ca2+ indicator GCaMP6f at a particular polarizer orientation (denoted in the top left corner). Indicator was expressed in both R7 and R8 photoreceptors. Bottom panel: responses for R7/R8 photoreceptors in three specific regions (i, ii and iii in top panel) at different e-vector angles. Paired R7/R8 photoreceptors exhibit opponent responses; the e-vector angles evoking peak responses in R7/R8 cells (arrowheads) shift linearly across receptor pairs. (D) Although neighboring regions of the DRA sample different sky regions, photoreceptors collectively sample all e-vector orientations. Top panel: optical axes (arrows) of DRA photoreceptors at distinct locations on the eye. Bottom panel: R7 photoreceptors are tuned to the full range of e-vector angles. Gray dots indicate microvillar orientations of R7 photoreceptors at distinct optical axes. Blue lines show preferred e-vector angle, measured via Ca2+ imaging. Adapted from Weir et al. (2016).
) of Ca2+ indicator GCaMP6f at a particular polarizer orientation (denoted in the top left corner). Indicator was expressed in both R7 and R8 photoreceptors. Bottom panel: responses for R7/R8 photoreceptors in three specific regions (i, ii and iii in top panel) at different e-vector angles. Paired R7/R8 photoreceptors exhibit opponent responses; the e-vector angles evoking peak responses in R7/R8 cells (arrowheads) shift linearly across receptor pairs. (D) Although neighboring regions of the DRA sample different sky regions, photoreceptors collectively sample all e-vector orientations. Top panel: optical axes (arrows) of DRA photoreceptors at distinct locations on the eye. Bottom panel: R7 photoreceptors are tuned to the full range of e-vector angles. Gray dots indicate microvillar orientations of R7 photoreceptors at distinct optical axes. Blue lines show preferred e-vector angle, measured via Ca2+ imaging. Adapted from Weir et al. (2016).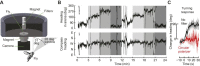
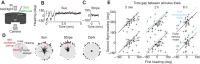
References
-
- Brines M. L. and Gould J. L. (1982). Skylight polarization patterns and animal orientation. J. Exp. Biol. 96, 69-91.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

