The insect central complex and the neural basis of navigational strategies
- PMID: 30728235
- PMCID: PMC6474401
- DOI: 10.1242/jeb.188854
The insect central complex and the neural basis of navigational strategies
Abstract
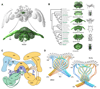
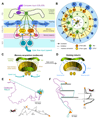
Oriented behaviour is present in almost all animals, indicating that it is an ancient feature that has emerged from animal brains hundreds of millions of years ago. Although many complex navigation strategies have been described, each strategy can be broken down into a series of elementary navigational decisions. In each moment in time, an animal has to compare its current heading with its desired direction and compensate for any mismatch by producing a steering response either to the right or to the left. Different from reflex-driven movements, target-directed navigation is not only initiated in response to sensory input, but also takes into account previous experience and motivational state. Once a series of elementary decisions are chained together to form one of many coherent navigation strategies, the animal can pursue a navigational target, e.g. a food source, a nest entrance or a constant flight direction during migrations. Insects show a great variety of complex navigation behaviours and, owing to their small brains, the pursuit of the neural circuits controlling navigation has made substantial progress over the last years. A brain region as ancient as insects themselves, called the central complex, has emerged as the likely navigation centre of the brain. Research across many species has shown that the central complex contains the circuitry that might comprise the neural substrate of elementary navigational decisions. Although this region is also involved in a wide range of other functions, we hypothesize in this Review that its role in mediating the animal's next move during target-directed behaviour is its ancestral function, around which other functions have been layered over the course of evolution.
Keywords: Insect brain; Motor control; Navigation; Neuroanatomy; Sensory integration.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures






References
-
- Bender JA, Pollack AJ, Ritzmann RE. Neural activity in the central complex of the insect brain is linked to locomotor changes. Curr Biol. 2010;20:921–926. - PubMed
-
- Byrne MJ, Dacke M, Nordström P, Scholtz CH, Warrant EJ. Visual cues used by ball-rolling dung beetles for orientation. J Comp Physiol A. 2003;189:411–418. - PubMed
-
- Cheng K, Srinivasan MV, Zhang S. Error is proportional to distance measured by honeybees: Weber’s law in the odometer. Anim Cogn. 1999;2:11–16.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

