Repeated human deciduous tooth-derived dental pulp cell reprogramming factor transfection yields multipotent intermediate cells with enhanced iPS cell formation capability
- PMID: 30728386
- PMCID: PMC6365514
- DOI: 10.1038/s41598-018-37291-2
Repeated human deciduous tooth-derived dental pulp cell reprogramming factor transfection yields multipotent intermediate cells with enhanced iPS cell formation capability
Abstract
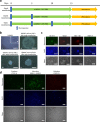
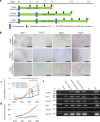
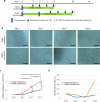
Human tissue-specific stem cells (hTSCs), found throughout the body, can differentiate into several lineages under appropriate conditions in vitro and in vivo. By transfecting terminally differentiated cells with reprogramming factors, we previously produced induced TSCs from the pancreas and hepatocytes that exhibit additional properties than iPSCs, as exemplified by very low tumour formation after xenogenic transplantation. We hypothesised that hTSCs, being partially reprogrammed in a state just prior to iPSC transition, could be isolated from any terminally differentiated cell type through transient reprogramming factor overexpression. Cytochemical staining of human deciduous tooth-derived dental pulp cells (HDDPCs) and human skin-derived fibroblasts following transfection with Yamanaka's factors demonstrated increased ALP activity, a stem cell marker, three weeks after transfection albeit in a small percentage of clones. Repeated transfections (≤3) led to more efficient iPSC generation, with HDDPCs exhibiting greater multipotentiality at two weeks post-transfection than the parental intact HDDPCs. These results indicated the utility of iPSC technology to isolate TSCs from HDDPCs and fibroblasts. Generally, a step-wise loss of pluripotential phenotypes in ESCs/iPSCs occurs during their differentiation process. Our present findings suggest that the reverse phenomenon can also occur upon repeated introduction of reprogramming factors into differentiated cells such as HDDPCs and fibroblasts.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Tamaoki, N. et al. Dental pulp cells for induced pluripotent stem cell banking. J. Dent. Res. 89, 773–778, 10.1177/0022034510366846 0022034510366846 [pii] (2010). - PubMed
-
- Inada, E. et al. Alkaline phosphatase and OCT3/4 as useful markers for predicting susceptibility of human deciduous teeth-derived dental pulp cells to reprogramming factor-induced iPS cells. J. Investig. Clin. Dent. 8 Epub ahead of print. 10.1111/jicd.12236 (2017). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

