General Principles of Neuronal Co-transmission: Insights From Multiple Model Systems
- PMID: 30728768
- PMCID: PMC6352749
- DOI: 10.3389/fncir.2018.00117
General Principles of Neuronal Co-transmission: Insights From Multiple Model Systems
Abstract
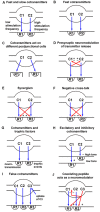
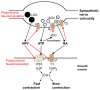
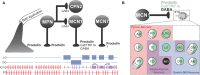
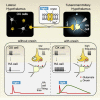
It is now accepted that neurons contain and release multiple transmitter substances. However, we still have only limited insight into the regulation and functional effects of this co-transmission. Given that there are 200 or more neurotransmitters, the chemical complexity of the nervous system is daunting. This is made more-so by the fact that their interacting effects can generate diverse non-linear and novel consequences. The relatively poor history of pharmacological approaches likely reflects the fact that manipulating a transmitter system will not necessarily mimic its roles within the normal chemical environment of the nervous system (e.g., when it acts in parallel with co-transmitters). In this article, co-transmission is discussed in a range of systems [from invertebrate and lower vertebrate models, up to the mammalian peripheral and central nervous system (CNS)] to highlight approaches used, degree of understanding, and open questions and future directions. Finally, we offer some outlines of what we consider to be the general principles of co-transmission, as well as what we think are the most pressing general aspects that need to be addressed to move forward in our understanding of co-transmission.
Keywords: colocalization; corelease; neuromodulation; neuropeptides; neurotransmitter complexity.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

