Increased PD-L1 expression in radioresistant HNSCC cell lines after irradiation affects cell proliferation due to inactivation of GSK-3beta
- PMID: 30728908
- PMCID: PMC6355177
- DOI: 10.18632/oncotarget.26542
Increased PD-L1 expression in radioresistant HNSCC cell lines after irradiation affects cell proliferation due to inactivation of GSK-3beta
Abstract
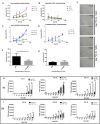
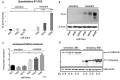
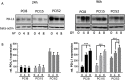
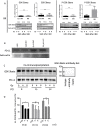
At present, targeting PD-1/PD-L1 axis for immune checkpoint inhibition has improved treatment of various tumor entities, including head and neck squamous cell carcinoma (HNSCC). However, one part of the patient cohort still shows little improvement or even hyperprogression. We established three radioresistant (RR) and three radiosensitive (RS) HNSCC cell lines. RR cells showed prolonged survival as well as delayed and diminished apoptosis after irradiation with vimentin expression but no E-cadherin expression, whereas RS cell lines died early and exhibited early apoptosis after irradiation and high vimentin expression. Here, we present results demonstrating differential basal PD-L1 gene and protein expression in RR and RS HNSCC cell lines. Moreover, we observed a radiation dose dependent increase of total PD-L1 protein expression in RR cell lines up to 96h after irradiation compared to non-irradiated (non-IRR) cells. We found a significant GSK-3beta phosphorylation, resulting in an inactivation, after irradiation of RR cell lines. Co-immunoprecipitation experiments revealed decreased interaction of GSK-3beta with PD-L1 in non-IRR compared to irradiated (IRR) RR cells leading to PD-L1 stabilization in RR cells. PD-L1 knockdown in RR cells showed a strong decrease in cell survival. In summary, our results suggest an irradiation dependent increase in basal PD-L1 expression in RR HNSCC cell lines via GSK-3beta inactivation.
Keywords: GSK-3beta; PD-L1; head and neck cancer; immune checkpoint; irradiation.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures

References
-
- Gevensleben H, Dietrich D, Golletz C, Steiner S, Jung M, Thiesler T, Majores M, Stein J, Uhl B, Muller S, Ellinger J, Stephan C, Jung K, et al. The Immune Checkpoint Regulator PD-L1 Is Highly Expressed in Aggressive Primary Prostate Cancer. Clinical Cancer Research. 2016;22:1969–77. doi: 10.1158/1078-0432.CCR-15-2042. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

