Effectiveness trials: critical data to help understand how respiratory medicines really work?
- PMID: 30728925
- PMCID: PMC6352944
- DOI: 10.1080/20018525.2019.1565804
Effectiveness trials: critical data to help understand how respiratory medicines really work?
Abstract
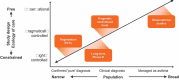
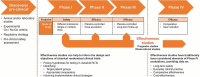
Most of the information about the benefits, safety aspects, and cost effectiveness of pharmacological treatment in the respiratory field has been obtained from traditional efficacy studies, such as randomised controlled trials (RCT). The highly controlled environment of an RCT does not always reflect everyday practice. The collection, analysis, and application of effectiveness data to generate Real World Evidence (RWE) through pragmatic trials or observational studies therefore has the potential to improve decision making by regulators, payers, and clinicians. Despite calls for more RWE, effectiveness data are not widely used in decision making in the respiratory field. Recent advances in data capture, curation, and storage combined with new analytical tools have now made it feasible for effectiveness data to become routine sources of evidence to supplement traditional efficacy data. In this paper, we will examine some of the current data gaps, diverse types of effectiveness data, look at proposed frameworks for the positioning of effectiveness data, as well as provide examples from therapeutic areas. We will give examples of both previous effectiveness studies and studies that are ongoing within the respiratory field. Effectiveness data hold the potential to address several evidentiary gaps related to the effectiveness, safety, and value of treatments in patients with respiratory diseases.
Keywords: COPD; Effectiveness; asthma; co-morbidity; efficacy RCT; evidence-base; levels of evidence; pragmatic trials; real-world evidence; respiratory disease.
Figures
References
-
- ABPI The vision for real world data – harnessing the opportunities in the UK. 2011[cited 2018 September] Available from: http://abpi.org.uk/media/1378/vision-for-real-world-data.pdf
-
- Holgate S, Bisgaard H, Bjermer L, et al. The Brussels declaration: the need for change in asthma management. Eur Respir J. 2008December;32(6):1433–7. PubMed PMID:19043008. - PubMed
Publication types
LinkOut - more resources
Full Text Sources