Childhood, adolescent and young adult non-Hodgkin lymphoma: current perspectives
- PMID: 30729513
- PMCID: PMC6897376
- DOI: 10.1111/bjh.15764
Childhood, adolescent and young adult non-Hodgkin lymphoma: current perspectives
Abstract
The 6th International Symposium on Childhood, Adolescent and Young Adult (CAYA) Non-Hodgkin Lymphoma (NHL) was held in Rotterdam, Netherlands, 26-29 September, 2018. This summary manuscript is a perspective on the presentations from the plenary scientific sessions, including wellness and survivorship, B-cell NHL, AYA lymphoma, translational NHL biology, lymphoma immunology, bone marrow transplantation and cell therapy, T/Natural Killer cell lymphoma, anaplastic large cell lymphoma, lymphoblastic lymphoma, novel lymphoma therapeutics and Hodgkin lymphoma. The symposium was attended by over 260 registrants from 42 different countries and included young, middle and senior investigators. Finally, the Angelo Rosolen, MD, Memorial Lecture was delivered by Alfred Reiter, MD.
Keywords: Childhood; adolescent; current perspectives; non-Hodgkin lymphoma; young adult.
© 2019 British Society for Haematology and John Wiley & Sons Ltd.
Figures
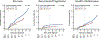




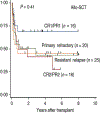
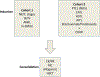
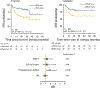






References
-
- Ansell SM (2017) Nivolumab in the treatment of Hodgkin lymphoma. Clinical Cancer Research, 23, 1623–1626. - PubMed
-
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA & Armand P. (2015) PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. New England Journal of Medicine, 372, 311–319. - PMC - PubMed
-
- Armstrong GT, Chen Y, Yasui Y, Leisenring W, Gibson TM, Mertens AC, Stovall M, Oeffinger KC, Bhatia S, Krull KR, Nathan PC, Neglia JP, Green DM, Hudson MM & Robison LL (2016) Reduction in late mortality among 5-year survivors of childhood cancer. New England Journal of Medicine, 374, 833–842. - PMC - PubMed
-
- Attarbaschi A. (2018) Diagnosis and treatment of rare B-NHL variants in children and adolescents. British Journal of Haematology, 182, 10. Abstract 19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

