Thoracoscopic thymectomy for juvenile myasthenia gravis
- PMID: 30729982
- PMCID: PMC6456483
- DOI: 10.1007/s00383-019-04441-0
Thoracoscopic thymectomy for juvenile myasthenia gravis
Abstract
Purpose: A randomized controlled trial of thymectomy in myasthenia gravis demonstrated improved clinical outcomes in adults, but data surrounding juvenile cases, especially those treated with minimally invasive approaches, are limited. Here, we review our experience with thoracoscopic thymectomy for juvenile myasthenia gravis (JMG) in the largest cohort to date.
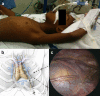
Methods: All cases of thymectomy for JMG in a single tertiary referral center between 2007 and 2018 were reviewed (N = 50). Patients underwent left thoracoscopic approach with extended dissection and without use of monopolar energy. Demographics, diagnostic criteria, and clinical classification, as well as surgical data were collected. Clinical status and medications were reviewed in follow-up.
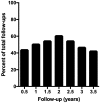
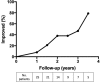
Results: The mean age at surgery was 10.5 ± 0.8 years. Ocular disease and generalized disease each comprised half of the cohort. No patients suffered complications or increased risk of morbidity or mortality with thymectomy. At any interval of follow-up through 3.5 years, 49.8% of patients were improved compared to their pre-operative presentation, and there was a significant trend towards decreased steroid use.
Conclusion: Thoracoscopic thymectomy is a safe treatment for juvenile myasthenia gravis in pediatric patients over a wide range of ages, body masses, and symptoms. Our experience adds evidence that pediatric patients likely benefit from thymectomy with improved clinical status and reduced medications.
Keywords: Juvenile myasthenia gravis; Minimally invasive surgery; Thoracoscopy; Thymectomy.
Conflict of interest statement
Conflict of interest
JFB is a consultant for Biogen, Avexis, PTC Therapeutics, Sarepta, Alexion, Cytokinetics, and Marathon, and is a speaker for Biogen. SWY is a member of the advisory board for PTC Therapeutics. The remaining authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The study was conducted under a waiver of consent (parental permission) per 45 CFR 46.116(d), a waiver of assent per 45 CFR 46.408(a), and a waiver of HIPAA authorization per 45 CFR 164.512(i)(2)(ii).
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

