Five-year outcome in the copaxone observatory: a nationwide cohort of patients with multiple sclerosis starting treatment with glatiramer acetate in France
- PMID: 30730008
- PMCID: PMC6420902
- DOI: 10.1007/s00415-019-09211-5
Five-year outcome in the copaxone observatory: a nationwide cohort of patients with multiple sclerosis starting treatment with glatiramer acetate in France
Abstract
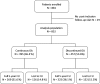
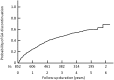
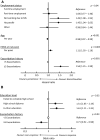
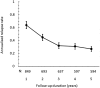
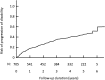
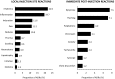
The benefits provided by disease-modifying treatments in multiple sclerosis have been demonstrated in clinical trials, but the extent to which they can be extrapolated to everyday care is less clear, as are the long-term benefits of treatment. The objective of this prospective observational cohort study performed in France was to evaluate the effectiveness and safety of glatiramer acetate in patients with relapsing-remitting multiple sclerosis over a 5-year period. All neurologists in France were invited to participate and enroll adult patients starting a first treatment with brand glatiramer acetate 20 mg. Given the observational nature of the study, no fixed study visits were imposed; consultations took place according to the investigator's normal practice. Occurrence of disease exacerbations and adverse events was documented and neurological disability evaluated with the EDSS at each consultation. Overall, 852 patients were analysable and 269 took glatiramer acetate continuously for 5 years. Median treatment duration was 3.4 years. Principal reasons for discontinuation were inadequate efficacy (38.9%), local tolerability (22.6%) and personal convenience (21.3%). Age, employment status, baseline EDSS score and number of previous exacerbations were variables associated with treatment persistence. The annualised exacerbation rate (5 years) was 0.41 [95% CI 0.39-0.44]; 316 patients (37.2%) remained exacerbation-free throughout. The risk of confirmed disability worsening (5 years) was 43.8% [95% CI 39.9-47.9%]. The most frequent adverse drug reactions were local injection site reactions (584 patients; 68.5%) and systemic immediate post-injection reactions (168 patients; 19.7%). Overall, these findings are consistent with those of previous clinical trials.
Keywords: Disease-modifying treatment; France; Glatiramer acetate; Observational study; Relapsing–remitting multiple sclerosis.
Conflict of interest statement
CLF has received consultancy or speakers fees from Biogen Idec, Merck Serono, Genzyme, Almirall, Allergan, Teva, Sanofi, Bayer Schering. AM, CPD and RB have received consultancy fees from Sanofi and Teva. TM and CL has received consultancy fees or speaking fees from Biogen Idec, Sanofi, Genzyme, Teva,Pharma, Bayer Schering, Merck Serono, Roche, Almirall and Novartis. FM is employee of Teva Pharma.
Figures
References
-
- Foulon S, Weill A, Maura G, Dalichampt M, Debouverie M, Moreau T. Prévalence de la sclérose en plaques en France en 2012 et mortalité associée en 2013 à partir des données du Sniiram-PMSI. Rev Epidemiol Santé Publique. 2015;63:S14–S22.
-
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. - DOI - PMC - PubMed
-
- Palace J, Duddy M, Bregenzer T, Lawton M, Zhu F, Boggild M, Piske B, Robertson NP, Oger J, Tremlett H, Tilling K, Ben-Shlomo Y, Dobson C. Effectiveness and cost-effectiveness of interferon beta and glatiramer acetate in the UK Multiple Sclerosis Risk Sharing Scheme at 6years: a clinical cohort study with natural history comparator. Lancet Neurol. 2015;14:497–505. doi: 10.1016/S1474-4422(15)00018-6. - DOI - PubMed
-
- Zhornitsky S, Greenfield J, Koch MW, Patten SB, Harris C, Wall W, Alikhani K, Burton J, Busche K, Costello F, Davenport JW, Jarvis SE, Lavarato D, Parpal H, Patry DG, Yeung M, Metz LM. Long-term persistence with injectable therapy in relapsingremitting multiple sclerosis: an 18-year observational cohort study. PLoS One. 2015;10:e0123824. doi: 10.1371/journal.pone.0123824. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

