Full Study Report of Andexanet Alfa for Bleeding Associated with Factor Xa Inhibitors
- PMID: 30730782
- PMCID: PMC6699827
- DOI: 10.1056/NEJMoa1814051
Full Study Report of Andexanet Alfa for Bleeding Associated with Factor Xa Inhibitors
Abstract
Background: Andexanet alfa is a modified recombinant inactive form of human factor Xa developed for reversal of factor Xa inhibitors.
Methods: We evaluated 352 patients who had acute major bleeding within 18 hours after administration of a factor Xa inhibitor. The patients received a bolus of andexanet, followed by a 2-hour infusion. The coprimary outcomes were the percent change in anti-factor Xa activity after andexanet treatment and the percentage of patients with excellent or good hemostatic efficacy at 12 hours after the end of the infusion, with hemostatic efficacy adjudicated on the basis of prespecified criteria. Efficacy was assessed in the subgroup of patients with confirmed major bleeding and baseline anti-factor Xa activity of at least 75 ng per milliliter (or ≥0.25 IU per milliliter for those receiving enoxaparin).
Results: Patients had a mean age of 77 years, and most had substantial cardiovascular disease. Bleeding was predominantly intracranial (in 227 patients [64%]) or gastrointestinal (in 90 patients [26%]). In patients who had received apixaban, the median anti-factor Xa activity decreased from 149.7 ng per milliliter at baseline to 11.1 ng per milliliter after the andexanet bolus (92% reduction; 95% confidence interval [CI], 91 to 93); in patients who had received rivaroxaban, the median value decreased from 211.8 ng per milliliter to 14.2 ng per milliliter (92% reduction; 95% CI, 88 to 94). Excellent or good hemostasis occurred in 204 of 249 patients (82%) who could be evaluated. Within 30 days, death occurred in 49 patients (14%) and a thrombotic event in 34 (10%). Reduction in anti-factor Xa activity was not predictive of hemostatic efficacy overall but was modestly predictive in patients with intracranial hemorrhage.
Conclusions: In patients with acute major bleeding associated with the use of a factor Xa inhibitor, treatment with andexanet markedly reduced anti-factor Xa activity, and 82% of patients had excellent or good hemostatic efficacy at 12 hours, as adjudicated according to prespecified criteria. (Funded by Portola Pharmaceuticals; ANNEXA-4 ClinicalTrials.gov number, NCT02329327.).
Copyright © 2019 Massachusetts Medical Society.
Figures


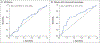
Comment in
-
Andexanet Alfa for Bleeding with Factor Xa Inhibitors.N Engl J Med. 2019 Jul 11;381(2):191. doi: 10.1056/NEJMc1906058. N Engl J Med. 2019. PMID: 31291532 No abstract available.
-
Andexanet Alfa for Bleeding with Factor Xa Inhibitors.N Engl J Med. 2019 Jul 11;381(2):191-192. doi: 10.1056/NEJMc1906058. N Engl J Med. 2019. PMID: 31291533 No abstract available.
-
FDA Approval of Andexanet Alfa Based on a Single-Arm Trial: If There Is a Villain, Who Is It?Ann Emerg Med. 2019 Nov;74(5):725-727. doi: 10.1016/j.annemergmed.2019.05.034. Ann Emerg Med. 2019. PMID: 31668248 No abstract available.
References
-
- The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363: 2499–510. - PubMed
-
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–91. - PubMed
-
- Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. - PubMed
-
- Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799–808. - PubMed
-
- Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319–30. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
