Performance of semi-automated hippocampal subfield segmentation methods across ages in a pediatric sample
- PMID: 30731245
- PMCID: PMC6524646
- DOI: 10.1016/j.neuroimage.2019.01.051
Performance of semi-automated hippocampal subfield segmentation methods across ages in a pediatric sample
Abstract
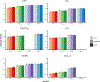
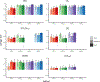
Episodic memory function has been shown to depend critically on the hippocampus. This region is made up of a number of subfields, which differ in both cytoarchitectural features and functional roles in the mature brain. Recent neuroimaging work in children and adolescents has suggested that these regions may undergo different developmental trajectories-a fact that has important implications for how we think about learning and memory processes in these populations. Despite the growing research interest in hippocampal structure and function at the subfield level in healthy young adults, comparatively fewer studies have been carried out looking at subfield development. One barrier to studying these questions has been that manual segmentation of hippocampal subfields-considered by many to be the best available approach for defining these regions-is laborious and can be infeasible for large cross-sectional or longitudinal studies of cognitive development. Moreover, manual segmentation requires some subjectivity and is not impervious to bias or error. In a developmental sample of individuals spanning 6-30 years, we assessed the degree to which two semi-automated segmentation approaches-one approach based on Automated Segmentation of Hippocampal Subfields (ASHS) and another utilizing Advanced Normalization Tools (ANTs)-approximated manual subfield delineation on each individual by a single expert rater. Our main question was whether performance varied as a function of age group. Across several quantitative metrics, we found negligible differences in subfield validity across the child, adolescent, and adult age groups, suggesting that these methods can be reliably applied to developmental studies. We conclude that ASHS outperforms ANTs overall and is thus preferable for analyses carried out in individual subject space. However, we underscore that ANTs is also acceptable and may be well-suited for analyses requiring normalization to a single group template (e.g., voxelwise analyses across a wide age range). Previous work has supported the use of such methods in healthy young adults, as well as several special populations such as older adults and those suffering from mild cognitive impairment. Our results extend these previous findings to show that ASHS and ANTs can also be used in pediatric populations as young as six.
Keywords: Development; High-resolution MRI; Reliability; Structural MRI; Volume.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


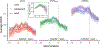


References
-
- Achenbach TM, 1991. Manual for the Child Behavior Checklist/4–18 and 1991 profile Department of Psychiatry, University of Vermont, Burlington, VT.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

