Disease progression in women with X-linked adrenoleukodystrophy is slow
- PMID: 30732635
- PMCID: PMC6367840
- DOI: 10.1186/s13023-019-1008-6
Disease progression in women with X-linked adrenoleukodystrophy is slow
Abstract
Background: Over 80% of women with X-linked adrenoleukodystrophy (ALD) develop spinal cord disease in adulthood for which treatment is supportive only. For future clinical trials quantitative data on disease progression rates are essential. Moreover, diagnosis can be challenging in ALD women, as the most important diagnostic biomarker is normal in 15-20%. Better biomarkers are needed. The purpose of this single centre cross-sectional follow-up study in women with ALD was to assess whether Expanded Disability Status Scale (EDSS), AMC Linear Disability Scale (ALDS) and Short Form (36) Health Survey (SF-36) can detect disease progression and to model the effect of age and duration of symptoms on the rate of progression. Moreover, we performed a pilot study to assess if a semi-targeted lipidomics approach can identify possible new diagnostic biomarkers.
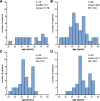
Results: In this study 46 women (baseline clinical data published by our group previously) were invited for a follow-up visit. Newly identified women at our center were also recruited. We analysed 65 baseline and 34 follow-up assessments. Median time between baseline and follow-up was 7.8 years (range 6.4-8.7). Mean age at baseline was 49.2 ± 14.2 years, at follow-up 55.4 ± 10.1. EDSS increased significantly (+ 0.08 points/year), but the other outcome measures did not. Increasing age and duration of symptoms were associated with more disability. For the pilot study we analysed plasma of 20 ALD women and 10 controls with ultra-high performance liquid chromatography coupled to high-resolution mass spectrometry, which identified 100 potential biomarker ratios with strong differentiating properties and non-overlapping data distributions between ALD women and controls.
Conclusions: Progression of spinal cord disease can be detected with EDSS, but not with ALDS or SF-36 after a follow-up period of almost 8 years. Moreover, age and the duration of symptoms seem positively associated with the rate of progression. Although a significant progression was measurable, it was below the rate generally conceived as clinically relevant. Therefore, EDSS, ALDS and SF-36 are not suitable as primary outcome measures in clinical trials for spinal cord disease in ALD women. In addition, a semi-targeted lipidomics approach can identify possible new diagnostic biomarkers for women with ALD.
Keywords: Adrenoleukodystrophy; Biomarkers; Progression; Spinal cord disease; Women.
Conflict of interest statement
Authors’ information (optional)
Not applicable.
Ethics approval and consent to participate
The local Institutional Review Board approved the study protocol (METC2015_079). Written informed consent was obtained from all participants.
Consent for publication
Consent for publication of raw data not obtained, but dataset is fully anonymous.
Competing interests
ICH – Consultant to Vertex.
MGWD – Reports no disclosures.
GEJ – Reports no disclosures.
MW – Reports no disclosures.
BMG – Reports no disclosures.
BTPT – Reports no disclosures.
SK – Received research grant from Bluebird Bio and is consultant to Vertex, Bluebird Bio and SwanBio Therapeutics.
ME – Received research grants from Vertex and Minoryx Therapeutics and is consultant to Vertex and Minoryx Therapeutics.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Moser HW, Smith KD, Watkins PA, Powers J, Moser AB, Adrenoleukodystrophy X-L. The metabolic and molecular bases of inherited disease. 2001. pp. 3257–3301.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

