Maternal obesity influences expression and DNA methylation of the adiponectin and leptin systems in human third-trimester placenta
- PMID: 30732639
- PMCID: PMC6367801
- DOI: 10.1186/s13148-019-0612-6
Maternal obesity influences expression and DNA methylation of the adiponectin and leptin systems in human third-trimester placenta
Abstract
Background: It is well established that obesity is associated with dysregulation of the ratio between the two major adipokines leptin and adiponectin. Furthermore, it was recently reported that maternal obesity has a significant impact on placental development. Leptin and adiponectin are present at the fetal-maternal interface and are involved in the development of a functional placenta. However, less is known about leptin and adiponectin's involvement in the placental alterations described in obese women. Hence, the objective of the present study was to characterize the placental expression and DNA methylation of these two adipokine systems (ligands and receptors) in obese women.
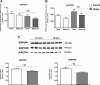
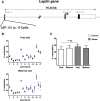
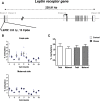
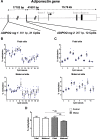
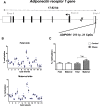
Results: Biopsies were collected from the fetal and maternal sides of third-trimester placenta in obese and non-obese (control) women. In both groups, leptin levels were higher on the fetal side than the maternal side, suggesting that this cytokine has a pivotal role in fetal growth. Secondly, maternal obesity (in the absence of gestational diabetes) was associated with (i) elevated DNA methylation of the leptin promoter on fetal side only, (ii) hypomethylation of the adiponectin promoter on the maternal side only, (iii) significantly low levels of leptin receptor protein (albeit in the absence of differences in mRNA levels and promoter DNA methylation), (iv) significantly low levels of adiponectin receptor 1 mRNA expression on the maternal side only, and (v) elevated DNA methylation of the adiponectin receptor 2 promoter on the maternal side only.
Conclusion: Our present results showed that maternal obesity is associated with the downregulation of both leptin/adiponectin systems in term placenta, and thus a loss of the beneficial effects of these two adipokines on placental development. Maternal obesity was also associated with epigenetic changes in leptin and adiponectin systems; this highlighted the molecular mechanisms involved in the placenta's adaptation to a harmful maternal environment.
Keywords: Adiponectin; DNA methylation; Leptin; Maternal obesity; Placenta.
Conflict of interest statement
Ethics approval and consent to participate
The present study was approved by the local investigational review board (
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

