Safety and immunogenicity of a varicella vaccine without human serum albumin (HSA) versus a HSA-containing formulation administered in the second year of life: a phase III, double-blind, randomized study
- PMID: 30732648
- PMCID: PMC6366055
- DOI: 10.1186/s12887-019-1425-7
Safety and immunogenicity of a varicella vaccine without human serum albumin (HSA) versus a HSA-containing formulation administered in the second year of life: a phase III, double-blind, randomized study
Abstract
Background: A new formulation of the live-attenuated varicella vaccine Varilrix (GSK) produced without human serum albumin (HSA) was developed to minimize a theoretical risk of transmission of infectious diseases. A previous study showed that the vaccine was immunologically non-inferior to the HSA-containing vaccine and well-tolerated in toddlers; low-grade fever was numerically higher in children receiving the vaccine without HSA, but the study lacked power to conclude on this difference.
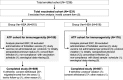
Methods: In this phase III, double-blind, multi-center study, healthy 12-23-month-olds were randomized (1:1) to receive two doses of the varicella vaccine without (Var-HSA group) or with HSA (Var + HSA group) at days 0 and 42. The primary objective compared safety of the vaccines in terms of incidence of fever > 39.0 °C in the 15-day period post-first vaccination. The objective was considered met if the upper limit of the 95% confidence interval for the between-group difference in the incidence of fever > 39.0 °C was ≤5% (Var-HSA group minus Var + HSA group). Safety, reactogenicity and immune responses were evaluated.
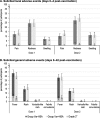
Results: Six hundred fifteen children in the Var-HSA group and 616 in the Var + HSA group received ≥1 vaccination. Fever > 39.0 °C was reported in 3.9 and 5.2% of participants in the Var-HSA and Var + HSA groups, with a between-group difference of - 1.29 (95% confidence interval: - 3.72-1.08); therefore, the primary objective was achieved. Fever rates post-each dose and the incidence of solicited local and general adverse events (AEs) were comparable between groups. Unsolicited AEs were reported for 43.9 and 36.5% of children in the Var-HSA group and 45.8 and 36.0% of children in the Var + HSA group, during 43 days post-dose 1 and 2, respectively. Serious AEs occurred in 2.1% (group Var-HSA) and 2.4% (group Var + HSA) of children, throughout the study. In a sub-cohort of 364 children, all had anti-varicella-zoster virus antibody concentrations ≥50 mIU/mL post-dose 2; comparable geometric mean concentrations were observed between the groups.
Conclusions: The varicella vaccine formulated without HSA did not induce higher rates of fever during the 15 day-post-vaccination period, as compared with the original HSA-containing vaccine. The two vaccines displayed similar safety and immunogenicity profiles in toddlers.
Trial registration: NCT02570126 , registered on 5 October 2015 (www.clinicaltrials.gov).
Keywords: Human serum albumin; Non-inferiority; Safety; Varicella vaccine.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by independent ethics committees/institutional review boards at each site (University of Tartu, Office of Research and Development; Landesarztekammer Baden-Wurttemberg Medical Board; National Institute of Pediatrics, Coyoacan; Medical Care and Research, Merida; Faculty of Medicine, Chulalongkorn University; NHS, Health Research Authority, North West-Liverpool East Research Ethics Committee) and conducted in accordance with provisions of the Declaration of Helsinki. Written informed consent was obtained from all children’s parents prior to enrolment in the study.
Consent for publication
Not applicable.
Competing interests
MLR, OH, CB, MP and PG are employees of the GSK group of companies, and OH and PG hold shares in the GSK group of companies as part of their employee remuneration. CB was employed by the GSK group of companies during the conduct of this study and is a current employee of Sanofi Pasteur. She holds shares in the GSK group of companies and Sanofi Pasteur. SNF declares that his institution received grants from the GSK group of companies for the conduct of this trial. SNF also declares support through his institution for the conduct of other trials (from the GSK group of companies, Sanofi-Pasteur, Pfizer, AstraZeneca, MedImmune, Alios, Ablynx and Merck). SNF declares that his institution received other support from Pfizer, AstraZeneca, MedImmune, Sanofi, Sequerius and Merck for advisory board participation. MDS declares that his institution received grants from the GSK group of companies for the conduct of this trial. MDS also declares support through his institution for the conduct of other trials (from the GSK group of companies, Sanofi-Pasteur, Pfizer, MedImmune, Novavax and Johnson and Johnson). CP, MA RW, KC, UB, JB, KH and CE MP have indicated they have no financial relationship or conflict of interest relevant to this article to disclose.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Varicella and herpes zoster vaccines WHO position paper, June 2014. Wkly Epidemiol Rec. 2014;89:265–287. - PubMed
-
- The European Agency for the Evaluation of Medicinal Products: Note for guidance on plasma-derived medicinal products. 2001. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin.... Accessed 14 Aug 2018.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

