Sound localization behavior in Drosophilamelanogaster depends on inter-antenna vibration amplitude comparisons
- PMID: 30733260
- PMCID: PMC6381992
- DOI: 10.1242/jeb.191213
Sound localization behavior in Drosophilamelanogaster depends on inter-antenna vibration amplitude comparisons
Abstract
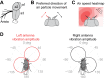
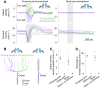
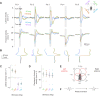
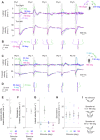
Drosophila melanogaster hear with their antennae: sound evokes vibration of the distal antennal segment, and this vibration is transduced by specialized mechanoreceptor cells. The left and right antennae vibrate preferentially in response to sounds arising from different azimuthal angles. Therefore, by comparing signals from the two antennae, it should be possible to obtain information about the azimuthal angle of a sound source. However, behavioral evidence of sound localization has not been reported in Drosophila Here, we show that walking D. melanogaster do indeed turn in response to lateralized sounds. We confirm that this behavior is evoked by vibrations of the distal antennal segment. The rule for turning is different for sounds arriving from different locations: flies turn toward sounds in their front hemifield, but they turn away from sounds in their rear hemifield, and they do not turn at all in response to sounds from 90 or -90 deg. All of these findings can be explained by a simple rule: the fly steers away from the antenna with the larger vibration amplitude. Finally, we show that these behaviors generalize to sound stimuli with diverse spectro-temporal features, and that these behaviors are found in both sexes. Our findings demonstrate the behavioral relevance of the antenna's directional tuning properties. They also pave the way for investigating the neural implementation of sound localization, as well as the potential roles of sound-guided steering in courtship and exploration.
Keywords: Auditory; Hearing; Insect; Johnston's organ; Phonotaxis.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures





References
-
- Atkins G. and Pollack G. S. (1987). Response properties of prothoracic, interganglionic, sound-activated interneurons in the cricket Teleogryllus oceanicus. J. Comp. Physiol. A 161, 681-693. 10.1007/BF00605009 - DOI
-
- Atkins G., Ligman S., Burghardt F. and Stout J. F. (1984). Changes in phonotaxis by the female cricket Acheta domesticus L. after killing identified acoustic interneurons. J. Comp. Physiol. A 154, 795-804. 10.1007/BF00610680 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

