Platelet glycoprotein VI and C-type lectin-like receptor 2 deficiency accelerates wound healing by impairing vascular integrity in mice
- PMID: 30733265
- PMCID: PMC6669159
- DOI: 10.3324/haematol.2018.208363
Platelet glycoprotein VI and C-type lectin-like receptor 2 deficiency accelerates wound healing by impairing vascular integrity in mice
Abstract
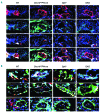
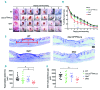
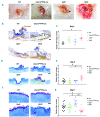
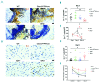
Platelets promote wound healing by forming a vascular plug and by secreting growth factors and cytokines. Glycoprotein (GP)VI and C-type lectin-like receptor (CLEC)-2 signal through a (hem)-immunoreceptor tyrosine-based activation motif, which induces platelet activation. GPVI and CLEC-2 support vascular integrity during inflammation in the skin through regulation of leukocyte migration and function, and by sealing sites of vascular damage. In this study, we investigated the role of impaired vascular integrity due to GPVI and/or CLEC-2 deficiency in wound repair using a full-thickness excisional skin wound model in mice. Transgenic mice deficient in both GPVI and CLEC-2 exhibited accelerated skin wound healing, despite a marked impairment in vascular integrity. The local and temporal bleeding in the skin led to greater plasma protein entry, including fibrinogen and clotting factors, was associated with increased fibrin generation, reduction in wound neutrophils and M1 macrophages, decreased level of tumor necrosis factor (TNF)-α, and enhanced angiogenesis at day 3 after injury. Accelerated wound healing was not due to developmental defects in CLEC-2 and GPVI double-deficient mice as similar results were observed in GPVI-deficient mice treated with a podoplanin-blocking antibody. The rate of wound healing was not altered in mice deficient in either GPVI or CLEC-2. Our results show that, contrary to defects in coagulation, bleeding following a loss of vascular integrity caused by platelet CLEC-2 and GPVI deficiency facilitates wound repair by increasing fibrin(ogen) deposition, reducing inflammation, and promoting angiogenesis.
Copyright© 2019 Ferrata Storti Foundation.
Figures


Similar articles
-
Dasatinib induces loss of vascular integrity and promotes cutaneous wound repair in mice.J Thromb Haemost. 2021 Dec;19(12):3154-3167. doi: 10.1111/jth.15499. Epub 2021 Aug 29. J Thromb Haemost. 2021. PMID: 34402195
-
Galectin-9 activates platelet ITAM receptors glycoprotein VI and C-type lectin-like receptor-2.J Thromb Haemost. 2022 Apr;20(4):936-950. doi: 10.1111/jth.15625. Epub 2022 Jan 6. J Thromb Haemost. 2022. PMID: 34936188
-
Significant Hypo-Responsiveness to GPVI and CLEC-2 Agonists in Pre-Term and Full-Term Neonatal Platelets and following Immune Thrombocytopenia.Thromb Haemost. 2018 Jun;118(6):1009-1020. doi: 10.1055/s-0038-1646924. Epub 2018 Apr 25. Thromb Haemost. 2018. PMID: 29695020 Free PMC article.
-
Platelet immunoreceptor tyrosine-based activation motif (ITAM) and hemITAM signaling and vascular integrity in inflammation and development.J Thromb Haemost. 2016 Apr;14(4):645-54. doi: 10.1111/jth.13250. Epub 2016 Feb 16. J Thromb Haemost. 2016. PMID: 26749528 Review.
-
Physiologic and pathophysiologic roles of interaction between C-type lectin-like receptor 2 and podoplanin: partners from in utero to adulthood.J Thromb Haemost. 2017 Feb;15(2):219-229. doi: 10.1111/jth.13590. Epub 2017 Feb 6. J Thromb Haemost. 2017. PMID: 27960039 Review.
Cited by
-
C-type lectin-like receptor 2: roles and drug target.Thromb J. 2024 Mar 19;22(1):27. doi: 10.1186/s12959-024-00594-8. Thromb J. 2024. PMID: 38504248 Free PMC article. Review.
-
Dasatinib Ointment Promotes Healing of Murine Excisional Skin Wound.ACS Pharmacol Transl Sci. 2023 Jun 9;6(7):1015-1027. doi: 10.1021/acsptsci.2c00245. eCollection 2023 Jul 14. ACS Pharmacol Transl Sci. 2023. PMID: 37470022 Free PMC article.
-
Intratumoral Platelets: Harmful or Incidental Bystanders of the Tumor Microenvironment?Cancers (Basel). 2022 Apr 27;14(9):2192. doi: 10.3390/cancers14092192. Cancers (Basel). 2022. PMID: 35565321 Free PMC article. Review.
-
A Perspective on How Fibrinaloid Microclots and Platelet Pathology May be Applied in Clinical Investigations.Semin Thromb Hemost. 2024 Jun;50(4):537-551. doi: 10.1055/s-0043-1774796. Epub 2023 Sep 25. Semin Thromb Hemost. 2024. PMID: 37748515 Free PMC article. Review.
-
Therapeutic targets and signaling mechanisms of dasatinib activity against radiation skin ulcer.Front Public Health. 2022 Nov 30;10:1031038. doi: 10.3389/fpubh.2022.1031038. eCollection 2022. Front Public Health. 2022. PMID: 36530656 Free PMC article.
References
-
- Drew AF, Liu H, Davidson JM, Daugherty CC, Degen JL. Wound-healing defects in mice lacking fibrinogen. Blood. 2001;97(12):3691–3698. - PubMed
-
- Hoffman M, Harger A, Lenkowski A, Hedner U, Roberts HR, Monroe DM. Cutaneous wound healing is impaired in hemophilia B. Blood. 2006;108(9):3053–3060. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

