HCMV trimer- and pentamer-specific antibodies synergize for virus neutralization but do not correlate with congenital transmission
- PMID: 30733288
- PMCID: PMC6397592
- DOI: 10.1073/pnas.1814835116
HCMV trimer- and pentamer-specific antibodies synergize for virus neutralization but do not correlate with congenital transmission
Abstract
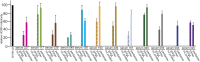
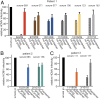
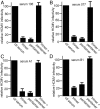
Human cytomegalovirus (HCMV) causes substantial disease in transplant patients and harms the development of the nervous system in babies infected in utero. Thus, there is a major focus on developing safe and effective HCMV vaccines. Evidence has been presented that a major target of neutralizing antibodies (NAbs) is the HCMV pentamer glycoprotein gH/gL/UL128-131. In some studies, most of the NAbs in animal or human sera were found to recognize the pentamer, which mediates HCMV entry into endothelial and epithelial cells. It was also reported that pentamer-specific antibodies correlate with protection against transmission from mothers to babies. One problem with the studies on pentamer-specific NAbs to date has been that the studies did not compare the pentamer to the other major form of gH/gL, the gH/gL/gO trimer, which is essential for entry into all cell types. Here, we demonstrate that both trimer and pentamer NAbs are frequently found in human transplant patients' and pregnant mothers' sera. Depletion of human sera with trimer caused reductions in NAbs similar to that observed following depletion with the pentamer. The trimer- and pentamer-specific antibodies acted in a synergistic fashion to neutralize HCMV and also to prevent virus cell-to-cell spread. Importantly, there was no correlation between the titers of trimer- and pentamer-specific NAbs and transmission of HCMV from mothers to babies. Therefore, both the trimer and pentamer are important targets of NAbs. Nevertheless, these antibodies do not protect against transmission of HCMV from mothers to babies.
Keywords: cell-to-cell spread; congenital; neutralizing antibodies; transplant; vaccines.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Arvin AM. National Vaccine Advisory Committee Vaccine development to prevent cytomegalovirus disease: Report from the National Vaccine Advisory Committee. Clin Infect Dis. 2004;39:233–239. - PubMed
-
- Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

