The Impact of FcγRI Binding on Immuno-PET
- PMID: 30733320
- PMCID: PMC6681692
- DOI: 10.2967/jnumed.118.223636
The Impact of FcγRI Binding on Immuno-PET
Abstract
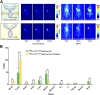
Antibodies are promising vectors for PET imaging. However, the high uptake of radioimmunoconjugates in nontarget tissues such as the liver and spleen hampers their performance as radiotracers. This off-target uptake can lead to suboptimal tumor-to-background activity concentration ratios, decreasing the contrast of images and reducing their diagnostic utility. A possible cause of this uptake is the sequestration of radioimmunoconjugates by immune cells bearing Fc-γ-receptors (FcγR) that bind to the Fc regions of antibodies. Methods: Since the heavy chain glycans influence the affinity of FcγR for the Fc domain, we set out to investigate whether radioimmunoconjugates with truncated glycans would exhibit altered binding to FcγRI and, in turn, improved in vivo performance. Using the HER2-targeting antibody trastuzumab, we synthesized a series of desferrioxamine-bearing immunoconjugates with differing glycosylation states and interrogated their FcγRI binding via surface plasmon resonance, enzyme-linked immunosorbent assay, and flow cytometry. Furthermore, we labeled these immunoconjugates with 89Zr and explored their biodistribution in athymic nude, NSG, and humanized NSG mice bearing human epidermal growth factor receptor 2-expressing human breast cancer xenografts. Results: We observed a strong correlation between the impaired in vitro FcγRI binding of deglycosylated immunoconjugates and significant decreases in the in vivo off-target uptake of the corresponding 89Zr-labeled radioimmunoconjugates (i.e., liver activity concentrations are reduced by ∼3.5-fold in humanized NSG mice). These reductions in off-target uptake were accompanied by concomitant increases in the tumoral activity concentrations of the glycoengineered radioimmunoconjugates, ultimately yielding improved tumor-to-healthy organ contrast and higher quality PET images. Conclusion: Our findings suggest that the deglycosylation of antibodies represents a facile strategy for improving the quality of immuno-PET in animal models as well as in certain patient populations.
Keywords: Fc receptor; Fc region; FcγRI; PET; glycans; radioimmunoconjugate.
© 2019 by the Society of Nuclear Medicine and Molecular Imaging.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
