Phospholipid regulation of innate immunity and respiratory viral infection
- PMID: 30733339
- PMCID: PMC6433062
- DOI: 10.1074/jbc.AW118.003229
Phospholipid regulation of innate immunity and respiratory viral infection
Abstract
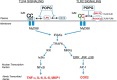
Toll-like receptors (TLRs) coupled to intracellular signaling cascades function as central elements of innate immunity that control transcription of numerous pro-inflammatory genes. Two minor anionic phospholipids present in the pulmonary surfactant complex, palmitoyl-oleoyl-phosphatidylglycerol (POPG) and phosphatidylinositol (PI), antagonize the cognate ligand activation of TLRs 2 and 4. The lipids block recognition of activating ligands by the TLRs, either directly or via the TLR4 coreceptors CD14 and MD2. Antagonism of TLR activation results in inhibition of the initiating step of the pro-inflammatory signaling pathways. Evidence for this mechanism of action comes from direct binding studies between CD14 and MD2 with POPG and PI. Additional evidence for this mechanism of antagonism also comes from monitoring the reduction of protein phosphorylation events that characterize the intracellular signaling by activated TLRs. The pathogenesis of respiratory syncytial virus (RSV) and influenza A virus (IAV) have been linked to TLR4 activation, and we examined the action of POPG and PI as potential antagonists of the pathology of these viruses. Surprisingly, POPG and PI dramatically curtail infection, in addition to inhibiting inflammatory sequelae associated with RSV and IAV infections. The mechanism of action by the lipids is disruption of virus particle binding to host cell plasma membrane receptors, required for viral uptake. The antagonism of activation of TLRs and virus binding to the alveolar epithelium by resident constituents of the pulmonary surfactant system suggests that POPG and PI function in homeostasis, to prevent inflammatory processes that result in reductions in gas exchange within the alveolar compartment.
Keywords: Toll-like receptors; antiviral agent; antivirals; immunology; inflammation; innate immunity; phospholipids; pulmonary surfactant; respiratory system; signaling; toll receptor; viral entry; virology.
© 2019 Voelker and Numata.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Kuronuma K., Mitsuzawa H., Takeda K., Nishitani C., Chan E. D., Kuroki Y., Nakamura M., and Voelker D. R. (2009) Anionic pulmonary surfactant phospholipids inhibit inflammatory responses from alveolar macrophages and U937 cells by binding the lipopolysaccharide-interacting proteins CD14 and MD-2. J. Biol. Chem. 284, 25488–25500 10.1074/jbc.M109.040832 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

