Separating host and microbiome contributions to drug pharmacokinetics and toxicity
- PMID: 30733391
- PMCID: PMC6533120
- DOI: 10.1126/science.aat9931
Separating host and microbiome contributions to drug pharmacokinetics and toxicity
Abstract
The gut microbiota is implicated in the metabolism of many medical drugs, with consequences for interpersonal variation in drug efficacy and toxicity. However, quantifying microbial contributions to drug metabolism is challenging, particularly in cases where host and microbiome perform the same metabolic transformation. We combined gut commensal genetics with gnotobiotics to measure brivudine drug metabolism across tissues in mice that vary in a single microbiome-encoded enzyme. Informed by these measurements, we built a pharmacokinetic model that quantitatively predicts microbiome contributions to systemic drug and metabolite exposure, as a function of bioavailability, host and microbial drug-metabolizing activity, drug and metabolite absorption, and intestinal transit kinetics. Clonazepam studies illustrate how this approach disentangles microbiome contributions to metabolism of drugs subject to multiple metabolic routes and transformations.
Copyright © 2019, American Association for the Advancement of Science.
Conflict of interest statement
Figures

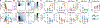
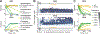
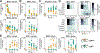


Comment in
-
Microbial chemistry gains fresh focus.Nature. 2019 Sep;573(7775):615-616. doi: 10.1038/d41586-019-02853-5. Nature. 2019. PMID: 31551561 No abstract available.
References
-
- Nishiyama T et al., Mechanism-Based Inactivation of Human Dihydropyrimidine Dehydrogenase by (E)-5-(2-Bromovinyl)uracil in the Presence of NADPH. Mol Pharmacol 57, 899–905 (2000). - PubMed
-
- Desgranges C et al., Effect of (E)-5-(2-bromovinyl)uracil on the catabolism and antitumor activity of 5-fluorouracil in rats and leukemic mice. Cancer Res. 46, 1094–1101 (1986). - PubMed
-
- Van Kuilenburg AB et al., Genotype and phenotype in patients with dihydropyrimidine dehydrogenase deficiency. Hum. Genet 104, 1–9 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

