Cellular signalling by primary cilia in development, organ function and disease
- PMID: 30733609
- PMCID: PMC6426138
- DOI: 10.1038/s41581-019-0116-9
Cellular signalling by primary cilia in development, organ function and disease
Abstract
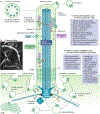
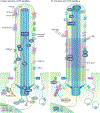
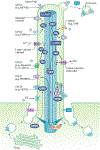
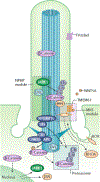
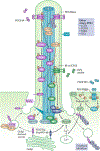
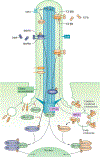
Primary cilia project in a single copy from the surface of most vertebrate cell types; they detect and transmit extracellular cues to regulate diverse cellular processes during development and to maintain tissue homeostasis. The sensory capacity of primary cilia relies on the coordinated trafficking and temporal localization of specific receptors and associated signal transduction modules in the cilium. The canonical Hedgehog (HH) pathway, for example, is a bona fide ciliary signalling system that regulates cell fate and self-renewal in development and tissue homeostasis. Specific receptors and associated signal transduction proteins can also localize to primary cilia in a cell type-dependent manner; available evidence suggests that the ciliary constellation of these proteins can temporally change to allow the cell to adapt to specific developmental and homeostatic cues. Consistent with important roles for primary cilia in signalling, mutations that lead to their dysfunction underlie a pleiotropic group of diseases and syndromic disorders termed ciliopathies, which affect many different tissues and organs of the body. In this Review, we highlight central mechanisms by which primary cilia coordinate HH, G protein-coupled receptor, WNT, receptor tyrosine kinase and transforming growth factor-β (TGFβ)/bone morphogenetic protein (BMP) signalling and illustrate how defects in the balanced output of ciliary signalling events are coupled to developmental disorders and disease progression.
Figures
References
-
- Satir P & Christensen ST Overview of structure and function of mammalian cilia. Annu Rev Physiol 69, 377–400 (2007). - PubMed
-
- Ciliopathies: A reference for clinicians. (eds. Kenny TD & Beales PL) 1–282 (Oxford University Press, Oxford, Oxford; 2014).
-
- Sorokin SP Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci 3, 207–230 (1968). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

