Transduction of Adeno-Associated Virus Vectors Targeting Hair Cells and Supporting Cells in the Neonatal Mouse Cochlea
- PMID: 30733670
- PMCID: PMC6353798
- DOI: 10.3389/fncel.2019.00008
Transduction of Adeno-Associated Virus Vectors Targeting Hair Cells and Supporting Cells in the Neonatal Mouse Cochlea
Abstract
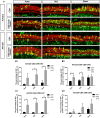
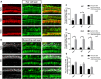
Adeno-associated virus (AAV) is the preferred vector for gene therapy of hereditary deafness, and different viral serotypes, promoters and transduction pathways can influence the targeting of AAV to different types of cells and the expression levels of numerous exogenous genes. To determine the transduction and expression patterns of AAV with different serotypes or promoters in hair cells and supporting cells in the neonatal mouse cochlea, we examined the expression of enhanced green fluorescent protein (eGFP) for five different types of AAV vectors [serotypes 2, 9, and Anc80L65 with promoter cytomegalovirus (CMV)-beta-Globin and serotypes 2 and 9 with promoter chicken beta-actin (CBA)] in in vitro cochlear explant cultures and we tested the transduction of AAV2/2-CBA, AAV2/9-CBA, and AAV2/Anc80L65-CMV by in vivo microinjection into the scala media of the cochlea. We found that each AAV vector had its own transduction and expression characteristics in hair cells and supporting cells in different regions of the cochlea. There was a tonotopic gradient for the in vitro transduction of AAV2/2-CBA, AAV2/9-CBA, AAV2/2-CMV, and AAV2/9-CMV in outer hair cells (OHCs), with more OHCs expressing eGFP at the base of the cochlea than at the apex. AAV2/2-CBA in vitro and AAV2/Anc80L65-CMV in vivo induced more supporting cells expressing eGFP at the apex than in the base. We found that AAV vectors with different promoters had different expression efficacies in hair cells and supporting cells of the auditory epithelium. The CMV-beta-Globin promoter could drive the expression of the delivered construct more efficiently in hair cells, while the CBA promoter was more efficient in supporting cells. The in vitro and in vivo experiments both demonstrated that AAV2/Anc80L65-CMV was a very promising vector for gene therapy of deafness because of its high transduction rates in hair cells. These results might be useful for selecting the appropriate vectors for gene delivery into different types of inner ear cells and thus improving the effectiveness of gene therapy.
Keywords: adeno-associated virus vectors; cochlea; gene therapy; hair cell; promoter; supporting cell.
Figures




References
-
- Bedrosian J. C., Gratton M. A., Brigande J. V., Tang W., Landau J., Bennett J. (2006). In vivo delivery of recombinant viruses to the fetal murine cochlea: transduction characteristics and long-term effects on auditory function. Mol. Ther. 14 328–335. 10.1016/j.ymthe.2006.04.003 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

