Unraveling the Regulation of Hepatic Gluconeogenesis
- PMID: 30733709
- PMCID: PMC6353800
- DOI: 10.3389/fendo.2018.00802
Unraveling the Regulation of Hepatic Gluconeogenesis
Abstract
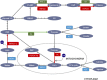
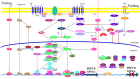
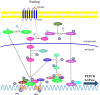
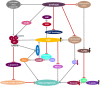
Hepatic gluconeogenesis, de novo glucose synthesis from available precursors, plays a crucial role in maintaining glucose homeostasis to meet energy demands during prolonged starvation in animals. The abnormally increased rate of hepatic gluconeogenesis contributes to hyperglycemia in diabetes. Gluconeogenesis is regulated on multiple levels, such as hormonal secretion, gene transcription, and posttranslational modification. We review here the molecular mechanisms underlying the transcriptional regulation of gluconeogenesis in response to nutritional and hormonal changes. The nutrient state determines the hormone release, which instigates the signaling cascades in the liver to modulate the activities of various transcriptional factors through various post-translational modifications like phosphorylation, methylation, and acetylation. AMP-activated protein kinase (AMPK) can mediate the activities of some transcription factors, however its role in the regulation of gluconeogenesis remains uncertain. Metformin, a primary hypoglycemic agent of type 2 diabetes, ameliorates hyperglycemia predominantly through suppression of hepatic gluconeogenesis. Several molecular mechanisms have been proposed to be metformin's mode of action.
Keywords: AMPK; acetylation; gluconeogenesis; hormone; metformin; methylation; transcription factor.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

