Biodegradation of feather waste by keratinase produced from newly isolated Bacillus licheniformis ALW1
- PMID: 30733740
- PMCID: PMC6353909
- DOI: 10.1016/j.jgeb.2018.05.005
Biodegradation of feather waste by keratinase produced from newly isolated Bacillus licheniformis ALW1
Abstract
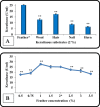
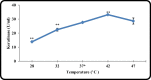
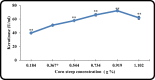
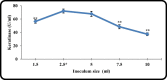
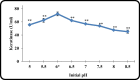
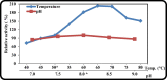
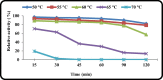
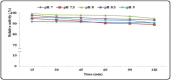
Keratinase are proteolytic enzymes which have gained much attention to convert keratinous wastes that cause huge environmental pollution problems. Ten microbial isolates were screened for their keratinase production. The most potent isolate produce 25.2 U/ml under static condition and was primarily identified by partial 16s rRNA gene sequence as Bacillus licheniformis ALW1. Optimization studies for the fermentation conditions increased the keratinase biosynthesis to 72.2 U/ml (2.9-fold). The crude extracellular keratinase was optimally active at pH 8.0 and temperature 65 °C with 0.7% soluble keratin as substrate. The produced B. licheniformis ALW1 keratinase exhibited a good stability over pH range from 7 to 9 and over a temperature range 50-60 °C for almost 90 min. The crude enzyme solution was able to degrade native feather up to 63% in redox free system.
Keywords: Bacillus licheniformisALW1; Enzymatic feather degradation; Feather waste; Keratinase; Optimization.
Figures



References
-
- Mazotto A., de Melo A., Macrae A., Rosado A., Peixoto R., Cedrola S. World J Microbiol Biotechnol. 2011;27:1355–1365. - PubMed
-
- Ire F.S., Onyenama A.C. J Adv Boil Biotechnol. 2017;13:1–13.
-
- Mabrouk M.E. World J Microbiol Biotechnol. 2008;24:2331–2338.
-
- Cedrola S., de Melo A., Mazotto A., Lins U., Zingali R., Rosado A. World J Microbiol Biotechnol. 2012;28:1259–1269. - PubMed
-
- Vasileva-Tonkova E., Gousterova A., Neshev G. Int Biodeterior Biodegradation. 2009;63:1008–1012.
LinkOut - more resources
Full Text Sources
